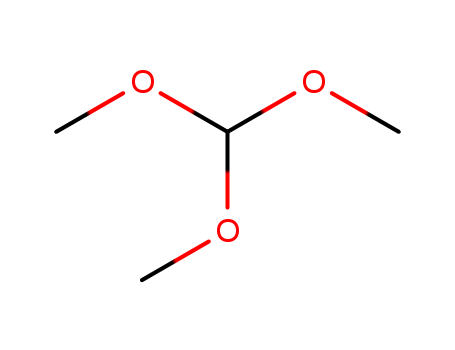
- Chemical Name:Trimethoxymethane
- CAS No.:149-73-5
- Deprecated CAS:251301-35-6
- Molecular Formula:C4H10O3
- Molecular Weight:106.122
- Hs Code.:29159080
- European Community (EC) Number:205-745-7
- NSC Number:147479
- UNII:XAM28819YJ
- DSSTox Substance ID:DTXSID7027122
- Nikkaji Number:J8.664I
- Wikipedia:Trimethyl_orthoformate
- Wikidata:Q408190
- ChEMBL ID:CHEMBL3187679
- Mol file:149-73-5.mol
Synonyms:Trimethoxymethane;TRIMETHYL ORTHOFORMATE;149-73-5;Methyl orthoformate;Methane, trimethoxy-;Orthoformic acid methyl ester;Orthoformic acid, trimethyl ester;Orthomravencan methylnaty;Methoxymethylal;Methylester kyseliny orthomravenci;trimethoxy-methane;NSC 147479;Orthoformic acid trimethyl ester;HSDB 1006;Orthomravencan methylnaty [Czech];Trimethylester kyseliny orthomravenci;EINECS 205-745-7;Orthoformic acid trimethyl;BRN 0969215;UNII-XAM28819YJ;CH(OCH3)3;Methylester kyseliny orthomravenci [Czech];AI3-23842;XAM28819YJ;DTXSID7027122;Trimethylester kyseliny orthomravenci [Czech];NSC-147479;EC 205-745-7;4-02-00-00022 (Beilstein Handbook Reference);trimethyl ortho formate;trimethoxy methane;trimethylorthoformat;trimethylorthoformate;trimetyl orthoformate;trirnethylorthoformate;MFCD00008483;trimethyl-orthoformate;trimethylortho-formate;tri-methyl orthoformate;trim ethyl orthoformate;trimethyl ortho-formate;trimethyl-ortho-formate;PERMA-FLO OF;(MeO)3CH;CH(OMe)3;HC(OMe)3;C(OC)(OC)OC;SCHEMBL6919;Trimethyl orthoformate, 99%;(CH3O)3CH;HC(OCH3)3;DTXCID307122;ortho-formic acid trimethylester;CHEMBL3187679;WLN: 1OYO1 & O1;ortho-formic acid trimethyl ester;PYOKUURKVVELLB-UHFFFAOYSA-;AMY41181;Tox21_200062;NSC147479;STL185659;TRIMETHYL ORTHOFORMATE [HSDB];AKOS000121043;NCGC00248510-01;NCGC00257616-01;BP-21313;CAS-149-73-5;LS-98477;Trimethyl orthoformate, anhydrous, 99.8%;O0068;ORTHOFORMIC ACID TRIMETHYL ESTER [MI];EN300-21653;A808947;Q408190;Q-201888;F0001-0529;(Trimethyl orthoformate)



 F,
F, Xi
Xi


