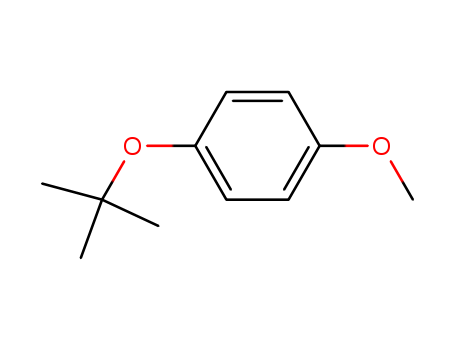
- Chemical Name:p-tert-Butoxyanisole
- CAS No.:15360-00-6
- Molecular Formula:C11H16 O2
- Molecular Weight:180.247
- Hs Code.:2909309090
- European Community (EC) Number:239-397-2
- UNII:2ESX92WW6E
- DSSTox Substance ID:DTXSID40165369
- Nikkaji Number:J99.566E
- Wikidata:Q83034516
- Mol file:15360-00-6.mol
Synonyms:p-tert-Butoxyanisole;15360-00-6;Benzene, 1-tert-butoxy-4-methoxy-;4-tert-butoxyanisole;Benzene, 1-(1,1-dimethylethoxy)-4-methoxy-;1-methoxy-4-[(2-methylpropan-2-yl)oxy]benzene;2ESX92WW6E;EINECS 239-397-2;4-t-butoxyanisole;4-(tert-Butoxy)anisole;UNII-2ESX92WW6E;t-butyl 4-methoxyphenyl ether;SCHEMBL5285766;1-tert-butoxy-4-methoxy-benzene;DTXSID40165369;1-tert-Butoxy-4-methoxybenzene #;MFCD26720802;SY298933;1-(1,1-Dimethylethoxy)-4-methoxybenzene