Products Categories
| CAS No.: | 10035-10-6 |
|---|---|
| Name: | Hydrogen bromide |
| Article Data: | 1337 |
| Molecular Structure: | |
|
|
|
| Formula: | HBr |
| Molecular Weight: | 80.9119 |
| Synonyms: | Hydrobromic acid;Acide bromhydrique;Acido bromhidrico;Anhydrous hydrobromic acid;Bromowodor;Bromure d'hydrogene anhydre;Bromuro de hidrogeno anhidro;Bromwasserstoff;Hydrogen bromide, anhydrous; |
| EINECS: | 233-113-0 |
| Density: | 1.49 g/mL at 25 °C(lit.) |
| Melting Point: | -87 °C(lit.) |
| Boiling Point: | -67 °C(lit.) |
| Flash Point: | 40 °C |
| Solubility: | soluble in water |
| Appearance: | colourless liquid with a strong irritating odour |
| Hazard Symbols: |
 C, C, Xi Xi
|
| Risk Codes: | 35-37-34-10-36/37/38 |
| Safety: | 26-45-7/9 |
| Transport Information: | UN 3265 8/PG 2 |
| PSA: | 0.00000 |
| LogP: | 0.95810 |
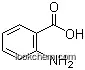
- 144851-82-1METHYL2-AMINO-3-FLUOROBENZOATE
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 173606-50-3BOC-10-AMINODECANOIC ACID
- 361456-36-2METHYL (R)-(+)-ISOCYANATO-3-PHENYLPROPI&
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 1219080-61-1IMIDAZOLE-2-BORONIC ACID
- 100367-55-32-Cyano-5-nitropyridine
- 100403-19-8Ceramides
- 10043-52-4Calcium chloride
- 1004-39-34,6-DIAMINO-2-MERCAPTOPYRIMIDINE
- 10045-25-71,4,7,10-TETRAAZACYCLODODECANE TETRAHYDROCHLORIDE
- 10048-98-3Barium hydrogen orthophosphate
- 10049-05-5Chromium(II) chloride
- 10049-23-7Tellurous(IV) acid
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
Chemistry
![]()
IUPAC Name: Hydrogen bromide
Molecular Formula: HBr
Molecular Weight: 80.91 g/mol
Canonical SMILES: Br
InChI: InChI=1/BrH/h1H
EINECS: 233-113-0
Classification Code: Ceiling (3 ppm); TWA 3 ppm (10 mg/m3)
Stability: Stable. Incompatible with strong bases, strong oxidizing agents, ammonia, ozone, fluorine, water, metals. Air and light sensitive.
Storage temperature: Refrigerator
Solubility: soluble
Water Solubility: soluble
Sensitive: Hygroscopic
XLogP3-AA: 1
Exact Mass: 79.926163
MonoIsotopic Mass: 79.926163
Heavy Atom Count: 1
Density: 1.49 g/mL at 25 °C(lit.)
Flash Point: 40 °C
Enthalpy of Vaporization: 19.92 kJ/mol
Boiling Point: -67 °C(lit.)
Melting Point: -87 °C(lit.)
Vapor density: 2.8 (vs air)
Vapor pressure: 334.7 psi ( 21 °C)
Vapour Pressure of Hydrogen bromide (CAS NO.10035-10-6): 20200 mmHg at 25 °C
Uses
Hydrogen bromide (CAS NO.10035-10-6) is mainly used for the production of inorganic bromides, especially the bromides of zinc, calcium, and sodium. It is a useful reagent for generating organobromine compounds.
Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LC50 | inhalation | 814ppm/1H (814ppm) | National Technical Information Service. Vol. PB214-270, | |
| rat | LC50 | inhalation | 2858ppm/1H (2858ppm) | National Technical Information Service. Vol. PB214-270, | |
| rat | LD50 | intraperitoneal | 76mg/kg (76mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 41(1), Pg. 105, 1976. |
Consensus Reports
Reported in EPA TSCA Inventory.
Safety Profile
A poison gas. A corrosive irritant to the eyes, skin, and mucous membranes. Reacts violently with F2, Fe2O3, NH3, O3. When heated to decomposition or in reaction with water or steam it emits toxic and corrosive fumes of Br− and HBr.
Hazard Codes:  C,
C, Xi
Xi
Risk Statements: 35-37-34-10-36/37/38
R35: Causes severe burns.
R37: Irritating to respiratory system.
R34: Causes burns.
R10: Flammable.
R36/37/38: Irritating to eyes, respiratory system and skin.
Safety Statements: 26-45-7/9
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S45: In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S7/9: Keep container tightly closed and in a well-ventilated place.
WGK Germany: 1
HazardClass: 8
PackingGroup of Hydrogen bromide (CAS NO.10035-10-6): II
Standards and Recommendations
OSHA PEL: CL 3 ppm
ACGIH TLV: CL 3 ppm; (Proposed: CL 2 ppm)
DFG MAK: 2 ppm (6.7 mg/m3)
DOT Classification: 8; Label: Corrosive (UN 1788); DOT Class: 2.3; Label: Poison Gas, Corrosive (UN 1048)
Analytical Methods
For occupational chemical analysis use NIOSH: Acids, Inorganic, 7903.
Specification
Hydrogen bromide (CAS NO.10035-10-6), its Synonyms are Hydrobromic acid ; Acide bromhydrique ; Acido bromhidrico ; Anhydrous hydrobromic acid ; Bromowodor ; Bromure d'hydrogene anhydre ; Bromuro de hidrogeno anhidro ; Bromwasserstoff ; Hydrogen bromide, anhydrous . It is colourless liquid with a strong irritating odour.