Products Categories
| CAS No.: | 11042-64-1 |
|---|---|
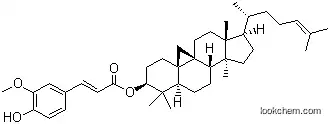
| Name: | gamma-Oryzanol |
| Molecular Structure: | |
 |
|
| Formula: | C40H58O4 |
| Molecular Weight: | 602.98 |
| Synonyms: | Gamma oryzanol;Oryzanol;CCRIS 4251;Gammariza;HI-Z;OZ;Oliver;UNII-SST9XCL51M;9,19-Cyclo-9beta-lanost-24-en-3beta-ol 4-hydroxy-3-methoxycinamate; |
| EINECS: | 244-285-1 |
| Density: | 1.1 g/cm3 |
| Melting Point: | 135-137 ºC |
| Boiling Point: | 663.2 ºC at 760 mmHg |
| Flash Point: | 193.8 ºC |
| Solubility: | soluble in acetone, chloroform; sparingly soluble in ethanol and n-heptane; insoluble in water |
| Appearance: | White or white crystalline powder, odourless |
| PSA: | 55.76000 |
| LogP: | 10.14740 |
- 144851-82-1METHYL2-AMINO-3-FLUOROBENZOATE
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 173606-50-3BOC-10-AMINODECANOIC ACID
- 361456-36-2METHYL (R)-(+)-ISOCYANATO-3-PHENYLPROPI&
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 1219080-61-1IMIDAZOLE-2-BORONIC ACID
- 55837-20-2Halofuginone
- 5337-93-91-Propanone,1-(4-methylphenyl)-
- 1116-76-31-Octanamine,N,N-dioctyl-
- 50-01-1Guanidine hydrochloride
- 121-69-7N,N-Dimethylaniline
- 600-21-5N-Methyl-DL-alanine
- 9002-18-0Agar
- 9000-71-9Casein
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
Specification
The γ-Oryzanol, with the CAS registry number 11042-64-1, has the product categories which are including Aromatic Esters; Nutritional Supplements. Besides, it is usually applied in curing many diseases, such as periodic psychosis, premenstrual tension, women's Menopause Syndrome, vascular headache, and dysfunction of autonomic nervoussystem.
The characteristics of this chemical are as below: (1)ACD/LogP: 12.85; (2)# of Rule of 5 Violations: 2; (3)ACD/LogD (pH 5.5): 12.85; (4)ACD/LogD (pH 7.4): 12.85; (5)#H bond acceptors: 4; (6)#H bond donors: 1; (7)#Freely Rotating Bonds: 10; (8)Polar Surface Area: 44.76; (9)Index of Refraction: 1.569; (10)Molar Refractivity: 178.62 cm3; (11)Molar Volume: 544.6 cm3; (12)Polarizability: 70.81×10-24 cm3; (13)Surface Tension: 46.2 dyne/cm; (14)Density: 1.1 g/cm3; (15)Flash Point: 193.8 °C; (16)Enthalpy of Vaporization: 101.08 kJ/mol; (17)Boiling Point: 663.2 °C at 760 mmHg; (18)Vapour Pressure: 3.38E-18 mmHg at 25°C; (19)Exact Mass: 602.43351; (20)MonoIsotopic Mass: 602.43351; (21)Topological Polar Surface Area: 55.8; (22)Heavy Atom Count: 44; (23)Complexity: 1150.
In adddition, you could convert the following datas into the molecular structure:
(1)Canonical SMILES: CC(CCC=C(C)C)C1CCC2(C1(CCC34C2CCC5C3(C4)CCC(C5(C)C)OC(=O)C=CC6=CC(=C(C=C6)O)OC)C)C
(2)Isomeric SMILES: C[C@H](CCC=C(C)C)[C@H]1CC[C@@]2([C@@]1(CC[C@]34[C@H]2CC[C@@H]5[C@]3(C4)CC[C@@H](C5(C)C)OC(=O)/C=C/C6=CC(=C(C=C6)O)OC)C)C
(3)InChI: InChI=1S/C40H58O4/c1-26(2)10-9-11-27(3)29-18-20-38(7)33-16-15-32-36(4,5)34(19-21-39(32)25-40(33,39)23-22-37(29,38)6)44-35(42)17-13-28-12-14-30(41)31(24-28)43-8/h10,12-14,17,24,27,29,32-34,41H,9,11,15-16,18-23,25H2,1-8H3/b17-13+/t27-,29-,32+,33+,34+,37-,38+,39-,40+/m1/s1
(4)InChIKey: FODTZLFLDFKIQH-FSVGXZBPSA-N
Below are the toxicity information of this chemical:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD | oral | > 5gm/kg (5000mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 6, Pg. 2401, 1978. | |
| guinea pig | LD50 | skin | 100mg/kg (100mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 7, Pg. 1295, 1979. | |
| mouse | LD | intramuscular | > 500mg/kg (500mg/kg) | Kiso to Rinsho. Clinical Report. Vol. 7, Pg. 2781, 1973. | |
| mouse | LD | intraperitoneal | > 10gm/kg (10000mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" SKIN AND APPENDAGES (SKIN): HAIR: OTHER | Kiso to Rinsho. Clinical Report. Vol. 7, Pg. 2781, 1973. |
| mouse | LD | subcutaneous | > 2500mg/kg (2500mg/kg) | Kiso to Rinsho. Clinical Report. Vol. 7, Pg. 2781, 1973. | |
| mouse | LD50 | intravenous | 811mg/kg (811mg/kg) | Drugs in Japan Vol. -, Pg. 297, 1995. | |
| mouse | LD50 | oral | > 25gm/kg (25000mg/kg) | Drugs in Japan Vol. -, Pg. 297, 1995. | |
| rabbit | LD | oral | > 5gm/kg (5000mg/kg) | GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 6, Pg. 2401, 1978. |
| rabbit | LD | subcutaneous | > 2500mg/kg (2500mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 6, Pg. 2401, 1978. | |
| rat | LD | intramuscular | > 500mg/kg (500mg/kg) | Kiso to Rinsho. Clinical Report. Vol. 7, Pg. 2781, 1973. | |
| rat | LD | subcutaneous | > 1gm/kg (1000mg/kg) | Kiso to Rinsho. Clinical Report. Vol. 7, Pg. 2781, 1973. | |
| rat | LD50 | intravenous | 382mg/kg (382mg/kg) | Drugs in Japan Vol. -, Pg. 297, 1995. | |
| rat | LD50 | oral | > 25gm/kg (25000mg/kg) | Drugs in Japan Vol. -, Pg. 297, 1995. | |
| rat | LD50 | skin | 100mg/kg (100mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 7, Pg. 1295, 1979. | |
| rat | LDLo | intraperitoneal | 1250mg/kg (1250mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" SKIN AND APPENDAGES (SKIN): HAIR: OTHER | Kiso to Rinsho. Clinical Report. Vol. 7, Pg. 2781, 1973. |

