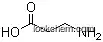Products Categories
| CAS No.: | 111-94-4 |
|---|---|
| Name: | 3,3'-I minobispropanenitrile |
| Article Data: | 41 |
| Molecular Structure: | |
|
|
|
| Formula: | C6H9 N3 |
| Molecular Weight: | 123.158 |
| Synonyms: | Propionitrile,3,3'-iminodi- (8CI); 3,3'-Iminobis(propionitrile);3,3'-Iminobis[propanenitrile]; 3,3'-Iminodipropionitrile; BBCE; Bis(2-cyanoethyl)amine;Bis(b-cyanoethyl)amine;Di(2-cyanoethyl)amine; Ethanamine, 2-cyano-N-(2-cyanoethyl)-; IDPN;Iminobis(propionitrile); N,N-Bis(2-cyanoethyl)amine; NSC 7770; b,b'-Iminodipropionitrile |
| EINECS: | 2, 5,8-Trioxanonane |
| Density: | 1.02 g/mL at 25 °C(lit.) |
| Melting Point: | -6 °C |
| Boiling Point: | 205 °C25 mm Hg(lit.) |
| Flash Point: | >230 °F |
| Appearance: | clear colorless to yellow liquid |
| Hazard Symbols: |
 Xi Xi
|
| Risk Codes: | 36/37/38 |
| Safety: | A poison by intraperitoneal route. Moderately toxic by ingestion and skin contact. Experimental teratogenic and reproductive effects. A skin and severe eye irritant. A storage hazard, may explode in a sealed container. When heated to decomposition it emits toxic fumes of NOx and CN−. See also NITRILES and AMINES. |
| PSA: | 59.61000 |
| LogP: | 0.79426 |
- 144851-82-1METHYL2-AMINO-3-FLUOROBENZOATE
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 173606-50-3BOC-10-AMINODECANOIC ACID
- 361456-36-2METHYL (R)-(+)-ISOCYANATO-3-PHENYLPROPI&
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 1219080-61-1IMIDAZOLE-2-BORONIC ACID
- Total:26 Page 1 of 1 1

What can I do for you?
Get Best Price
Chemistry
IUPAC Name: 3-(2-Cyanoethylamino)propanenitrile
Synonyms: 3,3'-Iminodipropionitrile ; 3,3'-Iminobis[propanenitrile] ; 3,3'-Iminodipropanenitrile ; 3,3'-Iminodipropionitrile ; Bis(.beta.-cyanoethyl)amine ; Di(2-cyanoethyl)amine ; Propanenitrile, 3,3'-iminobis-
CAS NO: 111-94-4
Molecular Formula of 3,3'-Iminodipropionitrile (CAS NO.111-94-4) : C6H9N3
Molecular Weight of 3,3'-Iminodipropionitrile (CAS NO.111-94-4) : 123.16
Molecular Structure of 3,3'-Iminodipropionitrile (CAS NO.111-94-4) : ![]()
EINECS: 203-922-3
Product Categories: Organics
Mol File: 111-94-4.mol
Index of Refraction: 1.452
Surface Tension: 43 dyne/cm
Density: 1 g/cm3
Flash Point: 149.2 °C
Enthalpy of Vaporization: 57.08 kJ/mol
Boiling Point: 328.4 °C at 760 mmHg
Vapour Pressure: 0.00019 mmHg at 25°C
Melting point: -6 °C
Stability: Stable, but moisture sensitive. Reaction with moisture may lead to a build up of pressure in sealed bottles. Combustible. Incompatible with strong oxidizing agents.
Appearance: 3,3'-Iminodipropionitrile (CAS NO.111-94-4) is colourless liquid.
Toxicity Data With Reference
| 1. | skn-rbt 500 mg/24H MLD | 85JCAE Prehled Prumyslove Toxikologie; Organicke Latky Marhold, J.,Prague, Czechoslovakia.: Avicenum,1986924. | ||
| 2. | eye-rbt 500 mg SEV | AJOPAA American Journal of Ophthalmology. 29 (1946),1363. | ||
| 3. | eye-rbt 500 mg/24H MLD | 85JCAE Prehled Prumyslove Toxikologie; Organicke Latky Marhold, J.,Prague, Czechoslovakia.: Avicenum,1986924. | ||
| 4. | orl-rat LD50:2700 mg/kg | JIHTAB Journal of Industrial Hygiene and Toxicology. 31 (1949),60. | ||
| 5. | ipr-mus LD50:200 mg/kg | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) AD277-689 . | ||
| 6. | skn-rbt LD50:2520 mg/kg | AMIHBC AMA Archives of Industrial Hygiene and Occupational Medicine. 10 (1954),61. |
Consensus Reports
Reported in EPA TSCA Inventory. Cyanide and its compounds are on the Community Right-To-Know List.
Safety Profile
A poison by intraperitoneal route. Moderately toxic by ingestion and skin contact. Experimental teratogenic and reproductive effects. A skin and severe eye irritant. A storage hazard, may explode in a sealed container. When heated to decomposition it emits toxic fumes of NOx and CN−. See also NITRILES and AMINES
Hazard Codes  Xi
Xi
Risk Statements 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements 26
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
WGK Germany 2
RTECS UG2975000
Specification
1.Air & Water Reactions: Water soluble. Moisture sensitive. Some 18-month old bottles of 33,3'-Iminodipropionitrile exploded, probably owing to slow hydrolysis and buildup of pressure. Solutions in water are unstable above pH 4. .
2.Reactivity Profile :Nitriles, such as 3,3'-Iminodipropionitrile , may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids.
3.Fire Hazard: 3,3'-Iminodipropionitrile is combustible.