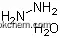Products Categories
| CAS No.: | 7664-41-7 |
|---|---|
| Name: | Ammonia |
| Article Data: | 3119 |
| Molecular Structure: | |
|
|
|
| Formula: | NH3 |
| Molecular Weight: | 17.0305 |
| Synonyms: | Ammoniagas;Ammonia-14N;Nitro-Sil;R 717;R 717 (ammonia);Refrigerent R717;Spiritof Hartshorn; |
| EINECS: | 231-635-3 |
| Density: | 1.023g/mLat 25°C |
| Melting Point: | - 77.73 °C, 195 K, -108 °F |
| Boiling Point: | - 33.34 °C, 240 K, -28 °F |
| Flash Point: | 52°F |
| Solubility: | in water: 47% (0 °C), 31% (25 °C),28% (50 °C) |
| Appearance: | Colourless gas with strong pungent odour |
| Hazard Symbols: |
 T; T;  N N
|
| Risk Codes: | 10-23-34-50 |
| Safety: | 26-45-36/37/39-16-9-61 |
| Transport Information: | UN 1005/2073 |
| PSA: | 3.24000 |
| LogP: | 0.32390 |
- 81281-59-67-Benzylideneaminotheophylline
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 914458-26-7[5-(2-fluorophenyl)-1-pentyl-1H-pyrrol-3-yl]-1-naphthalenyl-Methanone
- 894852-01-87-BROMO-2,2-DIMETHYL-2H-PYRIDO[3,2-B][1,4]OXAZIN-3(4H)-ONE
- 90221-55-92-bromo-5-methylbenzaldehyde
- 885590-99-82,3-DIFLUORO-4-IODOBENZALDEHYDE
- 97730-31-9(S)-4'-(2-Methylbutyl)Biphenyl-4-Carbonitrile

| Conditions | Yield |
|---|---|
| In water Electrolysis; Cu-cathode, in presence of H2SO4;; | 100% |
| With aluminium In water at elevated pressure;; | 0% |
| With aluminium In water only small amounts of NH3 in dild. HNO3 (5%-20%) at atmospheric pressure;; |

| Conditions | Yield |
|---|---|
| With water byproducts: CO; heating with H2O vapour to 300°C; | A 100% B n/a |
| With H2O |
- 180893-21-4
cis,trans-[WCl2(NNC5H2Me3-2,4,6)(C2H4)(PMe2Ph)2][BF4]

- 7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| With KOH In methanol byproducts: 2,4,6-trimethylpyridine; N2-atmosphere; excess KOH, stirring at room temp. for 1 h; collection of pyridine derivative (cold trap), colorimetry of NH3; | 100% |

- 7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| With KOH In methanol byproducts: 4-methoxypyridine; N2-atmosphere; excess KOH, stirring at room temp. for 1 h; collection of pyridine derivative (cold trap), colorimetry of NH3; | 100% |

- 7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| Alkaline conditions; | 100% |
- 16712-16-6, 64165-14-6, 65387-22-6, 70179-79-2, 540-69-2
ammonium formate

A
- 124-38-9
carbon dioxide

B
- 7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| With oxygen In water at 200℃; Catalytic behavior; Temperature; Flow reactor; Inert atmosphere; | A 100% B 100% |

| Conditions | Yield |
|---|---|
| at 130.0°C, 74.1 Torr equilibrium; | A 99.6% B 99.6% |
| at 130.0°C, 74.1 Torr equilibrium; | A 99.6% B 99.6% |
| at 54.8°C, 57.6 Torr equilibrium; | A 90.8% B 90.8% |
| at 54.8°C, 57.6 Torr equilibrium; | A 90.8% B 90.8% |

| Conditions | Yield |
|---|---|
| With [(C5H5)Mo(S2CH2)(S)(SH)Mo(C5H5)](OSO2CF3) In tetrahydrofuran Schlenk techniques; 10 equiv. of Mo complex in THF stirred at 25°C for 5 min under N2; N2 replaced by 1 atm of H2; W complex added portionwise; stirred at 25°C for 24 h and then at 55°C for 24 h; evapd. under reduced pressure; distillate trapped in dilute H2SO4 soln.;residue extd. with H2O, treated with activated charcoal, filtered throu gh Celite; | A 99% B 1% |


| Conditions | Yield |
|---|---|
| With catalyst: Ni(2+)Y zeolite In neat (no solvent) reduction of very dild. mixt. of NO/NO2 in gas mixt. of N2/H2 on zeolite catalyst (300°C reaction temp.); gas chromy. (dimethylsulfolane coated diatomite); | 99% |
| With catalyst: industrial nickel methanation catalyst In neat (no solvent) reduction of very dild. mixt. of NO/NO2 in gas mixt. of N2/H2 on zeolite catalyst (300°C reaction temp.); gas chromy. (dimethylsulfolane coated diatomite); | 99% |
| With catalyst: phthalocyanineNiY zeolite In neat (no solvent) reduction of very dild. mixt. of NO/NO2 in gas mixt. of N2/H2 on zeolite catalyst (230°C reaction temp.); gas chromy. (dimethylsulfolane coated diatomite); | 94% |
| With catalyst: NiY zeolite In neat (no solvent) reduction of very dild. mixt. of NO/NO2 in gas mixt. of N2/H2 on zeolite catalyst (450°C reaction temp.); gas chromy. (dimethylsulfolane coated diatomite); | 84% |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran Schlenk techniques; 2 equiv. of Mo complex in THF stirred at 25°Cfor 5 min under N2; N2 replaced by 1 atm of H2; W complex added portion wise; stirred at 25°C for 1 h; solvent removed under vac.; dissolved in THF-d8; not isolated; detd. by NMR spectra; | A 84% B 99% C 0% |

- 9016-45-9Poly(oxy-1,2-ethanediyl),a-(nonylphenyl)-w-hydroxy-
- 7782-49-2Selenium
- 8001-22-7Soybean oil
- 593-74-8Mercury, dimethyl-
- 7790-28-5Periodicacid (HIO4), sodium salt (1:1)
- 80-05-7Bisphenol A
- 61789-80-8Dimethyl di(hydrogenated tallow) ammonium chloride
- 61-52-91H-Indole-3-ethanamine,N,N-dipropyl-
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
- Total:17 Page 1 of 1 1
History
The Romans called the ammonium chloride deposits they collected from near the Temple of Jupiter Amun in ancient Libya 'sal ammoniacus' (salt of Amun) because of proximity to the nearby temple.Salts of ammonia have been known from very early times; thus the term Hammoniacus appears in the writings of Pliny, although it is not known whether the term is identical with the more modern sal-ammoniac.
In the form of sal-ammoniac, ammonia was known to the Muslim alchemists as early as the 8th century, first mentioned by Geber, and to the European alchemists since the 13th century, being mentioned by Albertus Magnus. It was also used by dyers in the Middle Ages in the form of fermented urine to alter the colour of vegetable dyes.
In the 15th century, Basilius Valentinus showed that ammonia could be obtained by the action of alkalis on sal-ammoniac. At a later period, when sal-ammoniac was obtained by distilling the hooves and horns of oxen and neutralizing the resulting carbonate with hydrochloric acid, the name "spirit of hartshorn" was applied to ammonia.
In 1774, gaseous ammonia was first isolated by Joseph Priestley and was termed by him alkaline air; however it was acquired by the alchemist Basil Valentine.
In 1785, Claude Louis Berthollet ascertained its composition.
The Haber process to produce ammonia from the nitrogen in the air was developed by Fritz Haber and Carl Bosch in 1909 and patented in 1910.
Specification
The Ammonia, with the CAS registry number 7664-41-7 and EINECS registry number 231-635-3, is a colourless gas with a characteristic pungent odour. And the molecular formula of this chemical is NH3. It is also hygroscopic, and belongs to the following product categories: Industrial/Fine Chemicals; refrigerants; Inorganics; Chemical Synthesis; Alternative Energy; AmidesChemical Synthesis; Compressed and Liquefied Gases; Materials for Hydrogen Storage; Synthetic Reagents. What's more, it is soluble in water, and should be stored at 0-6°C.
The Ammonia can be found in trace quantities in the atmosphere, and being produced from the putrefaction (decay process) of nitrogenous animal and vegetable matter. Ammonia and its salts can also be found in small quantities in rainwater. Ammonium chloride and ammonium sulfate can be found in volcanic districts. What's more, crystals of ammonium bicarbonate have been found in Patagonian guano.
The physical properties of Ammonia are as following: (1)#H bond acceptors: 1; (2)#H bond donors: 3; (3)#Freely Rotating Bonds: 0; (4)Polar Surface Area: 0 Å2; (5)Enthalpy of Vaporization: 23.33 kJ/mol; (6)Vapour Pressure: 5992.52 mmHg at 25°C.
Preparation of Ammonia: It can be preparation by the reaction between hydrogen and nitrogen (derived from process air), and the reaction will need magnetite catalyst under high pressure to form anhydrous liquid ammonia: 3 H2 + N2 → 2 NH3
Uses of Ammonia: Most ammonia is used as fertilizers either as its salts or as solutions. And it is directly or indirectly used as the precursor to most nitrogen-containing compounds. Household ammonia is a solution of NH3 in water (i.e., ammonium hydroxide) which is used as a general purpose cleaner for many surfaces. In addition, Ammonia solution(at 16-25%) is used in fermentation industry as a source of Nitrogen for the Micro-organisms as well as to adjust the pH during the Fermentation.
You should be cautious while dealing with this chemical. It is a kind of flammable chemical which is toxic by inhalation. It may also cause burns, and very toxic to aquatic organisms. Therefore, you had better take the following instructions: Wear suitable protective clothing, gloves and eye/face protection, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice; Keep container in a well-ventilated place; Keep away from sources of ignition - No smoking; In case of accident or if you feel unwell, seek medical advice immediately (show label where possible); Avoid release to the environment. Refer to special instructions safety data sheet.
You can still convert the following datas into molecular structure:
(1)SMILES: N
(2)InChI: InChI=1/H3N/h1H3
(3)InChIKey: QGZKDVFQNNGYKY-UHFFFAOYAF
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LC50 | inhalation | 7gm/m3/1H (7000mg/m3) | PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE) BEHAVIORAL: EXCITEMENT | Journal of Industrial Hygiene and Toxicology. Vol. 26, Pg. 29, 1944. |
| human | LCLo | inhalation | 5000ppm/5M (5000ppm) | Tabulae Biologicae. Vol. 3, Pg. 231, 1933. | |
| human | TCLo | inhalation | 20ppm (20ppm) | SENSE ORGANS AND SPECIAL SENSES: ULCERATED NASAL SEPTUM: OLFACTION SENSE ORGANS AND SPECIAL SENSES: CONJUNCTIVE IRRITATION: EYE LUNGS, THORAX, OR RESPIRATION: STRUCTURAL OR FUNCTIONAL CHANGE IN TRACHEA OR BRONCHI | Archiv fuer Gewerbepathologie und Gewerbehygiene. Vol. 13, Pg. 528, 1955. |
| mammal (species unspecified) | LCLo | inhalation | 5000ppm/5M (5000ppm) | Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 138, Pg. 65, 1928. | |
| man | LDLo | unreported | 132mg/kg (132mg/kg) | "Poisoning; Toxicology, Symptoms, Treatments," 2nd ed., Arena, J.M., Springfield, IL, C.C. Thomas, 1970Vol. 2, Pg. 73, 1970. | |
| man | TDLo | oral | 15uL/kg (0.015mL/kg) | GASTROINTESTINAL: CHANGE IN STRUCTURE OR FUNCTION OF ESOPHAGUS | American Journal of Emergency Medicine. Vol. 3, Pg. 320, 1985. |
| mouse | LC50 | inhalation | 4230ppm/1H (4230ppm) | BEHAVIORAL: TREMOR BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: ATAXIA | Federation Proceedings, Federation of American Societies for Experimental Biology. Vol. 41, Pg. 1568, 1982. |
| rabbit | LC50 | inhalation | 7gm/m3/1H (7000mg/m3) | PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE) BEHAVIORAL: EXCITEMENT | Journal of Industrial Hygiene and Toxicology. Vol. 26, Pg. 29, 1944. |
| rat | LC50 | inhalation | 2000ppm/4H (2000ppm) | "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 607, 1969. |