Products Categories
| CAS No.: | 140462-76-6 |
|---|---|
| Name: | Olopatadine hydrochloride |
| Article Data: | 18 |
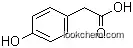
| Molecular Structure: | |
|
|
|
| Formula: | C21H23NO3·HCl |
| Molecular Weight: | 373.879 |
| Synonyms: | Dibenz[b,e]oxepin-2-aceticacid, 11-[3-(dimethylamino)propylidene]-6,11-dihydro-, hydrochloride, (11Z)-(9CI);ALO4943A;Allelock;KW 4679;Dibenz[b,e]oxepin-2-aceticacid, 11-[3-(dimethylamino)propylidene]-6,11-dihydro-, hydrochloride (1:1),(11Z)-;11-((Z)-3-(Dimethylamino)propylidene)-6,11-dihydrodibenz(b,e)oxepin-2-acetic acid, hydrochloride;(Z)-11-[3-(Dimethylamino)propylidene]-6,11-dihydrodibenz[b,e]oxepin-2-acetic acid hydrochloride; |
| EINECS: | 604-185-4 |
| Density: | == |
| Melting Point: | 242-245 °C |
| Boiling Point: | 523 °C at 760 mmHg |
| Flash Point: | 270.1 °C |
| Appearance: | White Solid |
| Hazard Symbols: | T,N |
| Risk Codes: | 25-50 |
| Safety: | 45-61 |
| PSA: | 49.77000 |
| LogP: | 4.39150 |
- 144851-82-1METHYL2-AMINO-3-FLUOROBENZOATE
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 173606-50-3BOC-10-AMINODECANOIC ACID
- 361456-36-2METHYL (R)-(+)-ISOCYANATO-3-PHENYLPROPI&
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE
- 60548-16-5
(11-oxo-6,11-dihydro-dibenzo[b,e]oxepin-2-yl)-acetic acid benzyl ester

- 27710-82-3
anhydrous 3-(dimethylamino)propyltriphenylphosphonium bromide hydrobromide

A
- 949141-22-4
(E )-11-[ 3-(dimethylamino)propylidene]-6,11-dihydrodibenz[b,e]oxepin-2-acetic acid hydrochloride

B
- 140462-76-6
olopatadine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: anhydrous 3-(dimethylamino)propyltriphenylphosphonium bromide hydrobromide With sodium hydride In tetrahydrofuran for 3h; Wittig Reaction; Heating / reflux; Stage #2: (11-oxo-6,11-dihydro-dibenzo[b,e]oxepin-2-yl)-acetic acid benzyl ester In tetrahydrofuran at 0 - 30℃; for 3h; Stage #3: With hydrogenchloride; water In di-isopropyl ether pH=2; | A 0.5% B 99% |

- 113806-05-6
olopatadine

- 140462-76-6
olopatadine hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water; acetone at 5 - 25℃; for 2 - 15h; Product distribution / selectivity; | 95.6% |
| With hydrogenchloride In water; acetone at 0 - 25℃; for 16 - 22h; Product distribution / selectivity; | 88.1% |
| With hydrogenchloride In water; acetone at 20 - 60℃; Product distribution / selectivity; | 81.2% |
| With hydrogenchloride In water | |
| With hydrogenchloride In tetrahydrofuran; water at 15 - 20℃; for 0.75 - 0.833333h; pH=0 - 1; Heating / reflux; |
- 55453-87-7
isoxepac

- 140462-76-6
olopatadine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: N-(3-chloropropyl)-N,N-dimethylamine hydrochloride With potassium bromide for 1h; Inert atmosphere; Stage #2: With N,N,N,N,N,N-hexamethylphosphoric triamide In dimethyl sulfoxide for 2h; Reflux; Stage #3: isoxepac In dimethyl sulfoxide at 20℃; for 1h; Reagent/catalyst; Solvent; | 90.5% |
- 113806-01-2
(Z)-11-<3-(dimethylamino)propylidene>-6,11-dihydrodibenzoxepin-2-acetic acid methyl ester

- 140462-76-6
olopatadine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: (Z)-11-<3-(dimethylamino)propylidene>-6,11-dihydrodibenzoxepin-2-acetic acid methyl ester With water; sodium hydroxide In methanol for 2h; Reflux; Stage #2: With hydrogenchloride In water pH=2; | 88.4% |
| Stage #1: (Z)-11-<3-(dimethylamino)propylidene>-6,11-dihydrodibenzoxepin-2-acetic acid methyl ester With sodium hydroxide In methanol; water at 20℃; for 3h; Stage #2: With hydrogenchloride In tetrahydrofuran; water for 0.0833333h; | 66% |

- 140462-76-6
olopatadine hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic anhydride In toluene at 100℃; for 6h; Sealed tube; | 79.5% |
- 55453-87-7
isoxepac

- 18355-96-9
(3-dimethylaminopropyl)-triphenylphosphoniumbromide

- 140462-76-6
olopatadine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: (3-dimethylaminopropyl)-triphenylphosphoniumbromide With sodium hydride In tetrahydrofuran at 20 - 22℃; for 1h; Stage #2: isoxepac With methanol In tetrahydrofuran at 0℃; for 2h; Reflux; | 60.6% |
- 113806-03-4
(Z)-11-(3-dimethylaminopropylidene)-6,11-dihydrodibenz[b,e] oxepin-2-acetic acid ethyl ester

- 140462-76-6
olopatadine hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In acetone for 10h; Product distribution / selectivity; Reflux; | 50% |
- 55453-87-7
isoxepac

- 27710-82-3
anhydrous 3-(dimethylamino)propyltriphenylphosphonium bromide hydrobromide

- 140462-76-6
olopatadine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: anhydrous 3-(dimethylamino)propyltriphenylphosphonium bromide hydrobromide With sodium hydride In tetrahydrofuran; mineral oil at 65℃; Inert atmosphere; Stage #2: isoxepac In tetrahydrofuran; mineral oil at 20℃; for 15h; Wittig Reaction; Stage #3: With hydrogenchloride In tetrahydrofuran; water; mineral oil pH=2.27; Product distribution / selectivity; | n/a |
| Stage #1: anhydrous 3-(dimethylamino)propyltriphenylphosphonium bromide hydrobromide With n-butyllithium In tetrahydrofuran at 0 - 5℃; for 1h; Inert atmosphere; Stage #2: isoxepac In tetrahydrofuran at 0℃; Reflux; Stage #3: With hydrogenchloride; acetic acid In tetrahydrofuran; water at 25 - 30℃; for 0.25h; Product distribution / selectivity; |
- 19070-16-7
3-(N,N-dimethylamino)propylmagnesium chloride

- 55453-87-7
isoxepac

- 140462-76-6
olopatadine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: 3-(N,N-dimethylamino)propylmagnesium chloride; isoxepac In tetrahydrofuran at 20℃; Grignard Reaction; Inert atmosphere; Stage #2: With ammonium chloride In tetrahydrofuran; water Stage #3: With hydrogenchloride In tetrahydrofuran; water at 20℃; for 17.75h; pH=~ 1; Reflux; | n/a |
- 55453-87-7
isoxepac

- 140462-76-6
olopatadine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: thionyl chloride / dichloromethane / 5 h / Reflux 2.1: dichloromethane / 0 - 20 °C / pH 9.5 - 10 3.1: tetrahydrofuran / 1 h / 0 - 5 °C 3.2: 0 - 35 °C 3.3: 0 - 5 °C / pH 9 - 10 4.1: hydrogenchloride; water / 90 - 95 °C 4.2: 0.5 h / 10 - 15 °C / pH 6.8 - 7.2 5.1: hydrogenchloride / chlorobenzene / 5 h / 80 - 85 °C View Scheme | |
| Multi-step reaction with 2 steps 1: toluene; tetrahydrofuran / 2.08 h / 5 - 18 °C 2: hydrogenchloride; acetic anhydride / toluene / 6 h / 100 °C / Sealed tube View Scheme |
- 56-41-7L-Alanine
- 21187-98-4Gliclazide
- 99-76-3Methylparaben
- 161798-01-25-Thiazolecarboxylicacid, 2-(3-formyl-4-hydroxyphenyl)-4-methyl-, ethyl ester
- 3380-34-5Triclosan
- 50-78-2Benzoicacid, 2-(acetyloxy)-
- 73183-34-3Bis(pinacolato)diboron
- 58-20-8Androst-4-en-3-one,17-(3-cyclopentyl-1-oxopropoxy)-, (17b)-
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin

What can I do for you?
Get Best Price
Specification
1. Introduction of Olopatadine hydrochloride
Olopatadine hydrochloride is one kind of white solid or white powder. The IUPAC Name of this chemical is 2-[(11E)-11-[3-(dimethylamino)propylidene]-6H-benzo[c][1]benzoxepin-2-yl]acetic acid hydrochloride. Besides, Olopatadine hydrochloride belongs to the product categories of Pharmaceutical material and intermeidates; APIs; Olopatadine; Amines; Heterocycles; Intermediates & Fine Chemicals; Pharmaceuticals. In addition, the Classification Code of Olopatadine hydrochloride is Anti-allergic. What's more, Olopatadine hydrochloride can soluble in water. Its systematic name is {(11Z)-11-[3-(Dimethylamino)propylidene]-6,11-dihydrodibenzo[b,e]oxepin-2-yl}acetic acid hydrochloride (1:1).
2. Properties of Olopatadine hydrochloride
Physical properties about Olopatadine hydrochloride are:
(1)ACD/LogP: 3.143; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.63; (4)ACD/LogD (pH 7.4): 0.64; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 3.71; (8)ACD/KOC (pH 7.4): 3.82; (9)#H bond acceptors: 4; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 5; (12)Polar Surface Area: 38.77 Å2; (13)Flash Point: 270.1 °C; (14)Enthalpy of Vaporization: 83.86 kJ/mol; (15)Boiling Point: 523 °C at 760 mmHg; (16)Vapour Pressure: 9.11E-12 mmHg at 25°C.
3. Structure Descriptors of Olopatadine hydrochloride
You can still convert the following datas into molecular structure:
(1)SMILES: Cl.O=C(O)Cc2ccc1OCc3c(C(\c1c2)=C\CCN(C)C)cccc3
(2)Std. InChI: InChI=1S/C21H23NO3.ClH/c1-22(2)11-5-8-18-17-7-4-3-6-16(17)14-25-20-10-9-15(12-19(18)20)13-21(23)24;/h3-4,6-10,12H,5,11,13-14H2,1-2H3,(H,23,24);1H/b18-8-;
(3)Std. InChIKey: HVRLZEKDTUEKQH-NOILCQHBSA-N
(4)Canonical SMILES: CN(C)CCC=C1C2=CC=CC=C2COC3=C1C=C(C=C3)CC(=O)O.Cl
(5)Isomeric SMILES: CN(C)CC/C=C\1/C2=CC=CC=C2COC3=C1C=C(C=C3)CC(=O)O.Cl
4. Uses of Olopatadine hydrochloride
This chemical is a dual acting histamine H1-receptor antagonist and mast cell stabilizer. It is used to treat itching associated with allergic conjunctivitis (eye allergies). It can be used as an antiallergic or antihistaminic. Olopatadine hydrochloride is an antihistimine but is usually prescribed as a nasal spray or an eyedrop.