Products Categories
| CAS No.: | 63-91-2 |
|---|---|
| Name: | L-Phenylalanine |
| Article Data: | 492 |
| Molecular Structure: | |
|
|
|
| Formula: | C9H11NO2 |
| Molecular Weight: | 165.192 |
| Synonyms: | beta-Phenylalnine, (-)-;Alanine, phenyl-;(-)-.beta.-Phenylalanine;(2S)-2-azaniumyl-3-phenyl-propanoate;(S)-Phenylalanine;alpha-Amino-beta-phenylpropionic acid, L-;(S)-alpha-Aminohydrocinnamic acid;beta-phenylalanine;L-.beta.-Phenylalanine;(2S)-2-amino-3-phenyl-propanoic acid;Phenylalamine;L-Antibiotic FN 1636;2-Amino-3-phenylpropionic acid, L-;Phenylalanine (USP);Hydrocinnamic acid, alpha-amino-;(L)-Phenylalanine;Alanine, phenyl-, L-;L-Phenylalanine (JP14); |
| EINECS: | 200-568-1 |
| Density: | 1.201 g/cm3 |
| Melting Point: | 270-275 °C (dec.)(lit.) |
| Boiling Point: | 307.5 °C at 760 mmHg |
| Flash Point: | 139.8 °C |
| Solubility: | water: 1-5 g/100 mL at 25 °C |
| Appearance: | white crystalline powder |
| Hazard Symbols: |
 C C
|
| Risk Codes: | 36/37/38-34 |
| Safety: | 22-24/25-37/39-45-36/37/39-27-26 |
| PSA: | 63.32000 |
| LogP: | 1.34130 |
- 81281-59-67-Benzylideneaminotheophylline
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE
- 68439-39-4Poly(oxy-1,2-ethanediyl), alpha-(2-ethylhexyl)-omega-hydroxy-,
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 717878-06-31-(4-fluorophenyl)-4-nitro-1H-imidazole
- 914458-26-7[5-(2-fluorophenyl)-1-pentyl-1H-pyrrol-3-yl]-1-naphthalenyl-Methanone
- 894852-01-87-BROMO-2,2-DIMETHYL-2H-PYRIDO[3,2-B][1,4]OXAZIN-3(4H)-ONE

| Conditions | Yield |
|---|---|
| With ammonium bicarbonate; water In dichloromethane for 48h; α-chymotrypsin; | 100% |
| With sodium hydroxide In water; ethyl acetate pH=9 - 10; | 93% |
| at 25℃; for 0.833333h; enzyme alcalase from Bacillus licheniforms; pH 8.2; |

| Conditions | Yield |
|---|---|
| With cell extract of Sphingomonas paucimobilis SC 16113 In water at 42℃; for 20h; Enzymatic reaction; | 100% |
| With hydrogen In ethanol for 0.5h; | 100% |
| With palladium on activated charcoal In methanol; ethyl acetate | 99% |

| Conditions | Yield |
|---|---|
| With ammonium bicarbonate; water In dichloromethane for 36h; α-chymotrypsin; | 100% |
| With Tris buffer; water at 50℃; Rate constant; | |
| With human valacyclovirase; water at 37℃; pH=7.4; Kinetics; Time; HEPES buffer; Enzymatic reaction; |
- 439912-45-5
((prop-2-yn-1-yloxy)carbonyl)-L-phenylalanine

- 63-91-2
L-phenylalanine

| Conditions | Yield |
|---|---|
| With resin bound tetrathiomolybdate In methanol at 28℃; for 1.5h; ultrasonic bath; | 100% |

| Conditions | Yield |
|---|---|
| With formate dehydrogenase; NAD; ammonium formate at 30℃; for 24h; PheDH; | 99% |
| With A-(modified B6)-B-<ω-amino(ethylamino)>-β-cyclodextrin In water at 30℃; for 5h; pH 8.0 (phosphate buffer); Yield given; | |
| With formate dehydrogenase; NAD; ammonium formate at 30℃; for 24h; Mechanism; buffer (pH 8.5); other oxo-acids; PheDH; |
- 108321-83-1
phenylalanine amide hydrochloride

- 63-91-2
L-phenylalanine

| Conditions | Yield |
|---|---|
| With pyridoxal 5'-phosphate monohydrate; cobalt(II) chloride In aq. buffer at 40℃; for 4h; pH=7.0; Enzymatic reaction; | 99% |

| Conditions | Yield |
|---|---|
| With pepsin immobilized on terephthalaldehyde functionalized chitosan magnetic nanoparticle In acetonitrile at 20℃; for 48h; pH=2; | 98% |
| With hydrogenchloride | |
| With hydrogen bromide |

| Conditions | Yield |
|---|---|
| With ammonium formate; tris hydrochloride; nicotinamide adenine dinucleotide; formate dehydrogenase; phenylalanine dehydrogenase In water at 30℃; for 24h; | 98% |
| With ammonium formate; tris hydrochloride; nicotinamide adenine dinucleotide; phenylalanine and formate dehydrogenase In water at 30℃; for 24h; Equilibrium constant; pH = 8.5; | 98% |
| With L-glutamic acid; pyridoxal 5'-phosphate In water at 40℃; for 12h; E.coli Aspartate transaminase, pH 8; | 84% |
| With L-glutamic acid; E.coli Aspartate transaminase; pyridoxal 5'-phosphate at 40℃; for 12h; enzyme kinetics; pH 8; Miachaelis constant (Km); maximum velocity constant (vmax); further α-keto acids; | 84% |
| With L-glutamine; human glutamine transaminase K at 37℃; pH=7.4; aq. phosphate buffer; Enzymatic reaction; |
- 631921-67-0
((S)-1-Cyano-2-phenyl-ethyl)-carbamic acid methyl ester

- 63-91-2
L-phenylalanine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water for 7h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| With hydrogen; nickel In water; acetic acid under 25857.4 Torr; | 97% |
- 103577-45-3Lansoprazole
- 71751-41-2Abamectin
- 97240-79-4Topiramate
- 1643-19-21-Butanaminium,N,N,N-tributyl-, bromide (1:1)
- 50-23-7Hydrocortisone
- 73590-58-61H-Benzimidazole, 5-methoxy-2-(((4-methoxy-3,5-dimethyl-2-pyridinyl)methyl)sulfinyl)-
- 114772-54-2[1,1'-Biphenyl]-2-carbonitrile,4'-(bromomethyl)-
- 9007-28-7Chondroitin sulfate
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
History
The genetic codon for phenylalanine was first discovered by J. Heinrich Matthaei and Marshall W. Nirenberg in 1961. This discovery helped to establish the nature of the coding relationship that links information stored in genomic nucleic acid with protein expression in the living cell.
Consensus Reports
Reported in EPA TSCA Inventory.
Specification
1. Introduction of L-Phenylalanine
L-Phenylalanine, with the IUPAC Name of (2S)-2-amino-3-phenylpropanoic acid, is one kind of white crystalline powder. This chemical is incompatible with strong oxidizing agents, and it belongs to the Product Categories which include Food and Feed Additive;Amino ACIDS SERIES;API intermediates;Phenylalanine [Phe, F];Amino Acids and Derivatives;Amino Acids 13C, 2H, 15N;alpha-Amino Acids;Amino Acids;Biochemistry;Nutritional Supplements;L-Amino Acids;Amino Acids;Amino Acids & Derivatives.
2. Properties of L-Phenylalanine
L-Phenylalanine has the following datas: (1)Index of Refraction: 1.576; (2)Molar Refractivity: 45.49 cm3; (3)Molar Volume: 137.4 cm3; (4)Polarizability: 18.03×10-24cm3; (5)Surface Tension: 53.5 dyne/cm; (6)Density: 1.201 g/cm3; (7)Flash Point: 139.8 °C; (8)Enthalpy of Vaporization: 57.87 kJ/mol; (9)Melting Point: 270-275 °C (dec.)(lit.); (10)Boiling Point: 307.5 °C at 760 mmHg; (11)Vapour Pressure: 0.000313 mmHg at 25°C; (12)Water Solubility: 1-5 g/100 mL at 25 oC.
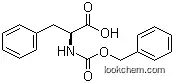
3. Structure Descriptors of L-Phenylalanine
You could convert the following datas into the molecular structure:
InChI:InChI=1S/C9H11NO2/c10-8(9(11)12)6-7-4-2-1-3-5-7/h1-5,8H,6,10H2,(H,11,12)/t8-/m0/s1
InChIKey:InChIKey=COLNVLDHVKWLRT-QMMMGPOBSA-N
Smiles:c1(C[C@@H](C(O)=O)N)ccccc1
4. Toxicity of L-Phenylalanine
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | > 1322mg/kg (1322mg/kg) | Yakugaku Zasshi. Journal of Pharmacy. Vol. 97, Pg. 1117, 1977. | |
| rat | LD50 | intraperitoneal | 5287mg/kg (5287mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Archives of Biochemistry and Biophysics. Vol. 58, Pg. 253, 1955. |
5. Safety Information of L-Phenylalanine
Mildly toxic by intraperitoneal route. An experimental teratogen. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Hazard Codes:
 C: Corrosive
C: Corrosive Risk Statements: 36/37/38-34
36/37/38: Irritating to eyes, respiratory system and skin
34: Causes burns
Safety Statements: 22-24/25-37/39-45-36/37/39-27-26
22: Do not breathe dust
24/25: Avoid contact with skin and eyes
37/39: Wear suitable gloves and eye/face protection
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
36/37/39: Wear suitable protective clothing, gloves and eye/face protection
27: Take off immediately all contaminated clothing
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
F 10: Keep under argon
WGK Germany: 3
6. Use of L-Phenylalanine
L-Phenylalanine is an electrically-neutral amino acid, one of the twenty common amino acids used to biochemically form proteins, coded for by DNA.