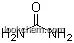Products Categories
| CAS No.: | 76-74-4 |
|---|---|
| Name: | Pentobarbitone |
| Article Data: | 15 |
| Molecular Structure: | |
|
|
|
| Formula: | C11H18N2O3 |
| Molecular Weight: | 226.276 |
| Synonyms: | 2,4,6(1H,3H,5H)-Pyrimidinetrione, 5-ethyl-5-(1-methylbutyl)-;5-Ethyl-5-(1-methylbutyl)-2,4,6(1H,3H,5H)-pyrimidinetrione;5-Ethyl-5-(1-methylbutyl)barbituric acidcid;5-ethyl-5-(1-methylbutyl)-barbituricaci;5-Ethyl-5-(1-methylbutyl)malonylurea;5-Ethyl-5-(sec-pentyl)barbituric acid;5-ethyl-5-(sec-pentyl)barbituricacid;6(1h,3h,5h)-pyrimidinetrione,5-ethyl-5-(1-methylbutyl)-4 |
| EINECS: | 200-983-8 |
| Density: | 1.081 g/cm3 |
| Melting Point: | 129.5°C |
| Boiling Point: | 367.89°C (rough estimate) |
| Flash Point: | 9℃ |
| Solubility: | soluble in water |
| Hazard Symbols: |
 F; F;  T T
|
| Risk Codes: | 25-63-39/23/24/25-36/38-23/24/25-11 |
| Safety: | 7-16-36/37-45-36/37/39-22 |
| Transport Information: | UN 2811 |
| PSA: | 75.27000 |
| LogP: | 1.84260 |
- 81281-59-67-Benzylideneaminotheophylline
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 914458-26-7[5-(2-fluorophenyl)-1-pentyl-1H-pyrrol-3-yl]-1-naphthalenyl-Methanone
- 894852-01-87-BROMO-2,2-DIMETHYL-2H-PYRIDO[3,2-B][1,4]OXAZIN-3(4H)-ONE
- 90221-55-92-bromo-5-methylbenzaldehyde
- 885590-99-82,3-DIFLUORO-4-IODOBENZALDEHYDE
- 97730-31-9(S)-4'-(2-Methylbutyl)Biphenyl-4-Carbonitrile
 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
Specification
The Pentobarbital, with the CAS registry number 76-74-4 and EINECS registry number 200-983-8, has the systematic name of 5-ethyl-5-(pentan-2-yl)pyrimidine-2,4,6(1H,3H,5H)-trione. And the molecular formula of this chemical is C11H18N2O3. In addition, it should be stored at 2-8°C. Besides, it is a short-acting barbiturate that is effective as a sedative and hypnotic (but not as an anti-anxiety) agent and is usually given orally.
The physical properties of Pentobarbital are as following: (1)ACD/LogP: 2.05; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.05; (4)ACD/LogD (pH 7.4): 1.93; (5)ACD/BCF (pH 5.5): 21.31; (6)ACD/BCF (pH 7.4): 16.12; (7)ACD/KOC (pH 5.5): 310.5; (8)ACD/KOC (pH 7.4): 234.9; (9)#H bond acceptors: 5; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 57.69 Å2; (13)Index of Refraction: 1.465; (14)Molar Refractivity: 57.89 cm3; (15)Molar Volume: 209.1 cm3; (16)Polarizability: 22.95×10-24cm3; (17)Surface Tension: 34.6 dyne/cm; (18)Density: 1.081 g/cm3.
Preparation of Pentobarbital: Dissolve 26.7 g of clean metallic sodium in 400 g of anhydrous (dry) ethanol. Add a solution of 100 g of 1-methyl butyl-ethyl malonic ester and 37.2 g of dry urea. Heat the mixture for 4 to 6 hours in an autoclave, or refluxe for 20 to 40 hours. The alcohol is then removed by distillation. The residue is dissolved in water and this aqueous solution is acidified with hydrochloric acid. The precipitated product is filtered, washed with cold water, and recrystallized from boiling water.
Uses of Pentobarbital: Its FDA approved human uses include treatment of seizures and preoperative (and other) sedation. It is also approved as a short-term hypnotic. And off-label uses of pentobarbital include reduction of intracranial pressure in Reye's syndrome, traumatic brain injury and induction of coma in cerebral ischemia patients. In addition, it is also used in combination with complementary agents such as phenytoin, in commercial animal euthanasia injectable solutions. What's more, It used for physician-assisted suicide.
You should be cautious while dealing with this chemical. It is a kind of flammable chemical, and has the danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed. It also irritates eyes, respiratory system and skin. In addition, it has possible risk of harm to the unborn child. Therefore, you had better take the following instructions: Wear suitable protective clothing, gloves and eye/face protection; Keep container tightly closed; Keep away from sources of ignition - No smoking; Do not breathe dust; In case of accident or if you feel unwell, seek medical advice immediately (show label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: O=C1NC(=O)NC(=O)C1(C(C)CCC)CC
(2)InChI: InChI=1/C11H18N2O3/c1-4-6-7(3)11(5-2)8(14)12-10(16)13-9(11)15/h7H,4-6H2,1-3H3,(H2,12,13,14,15,16)
(3)InChIKey: WEXRUCMBJFQVBZ-UHFFFAOYAD
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LD50 | intravenous | 72mg/kg (72mg/kg) | Federation Proceedings, Federation of American Societies for Experimental Biology. Vol. 28, Pg. 1479, 1969. | |
| dog | LD50 | intravenous | 50mg/kg (50mg/kg) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 141, Pg. 83, 1963. |
| human | LDLo | oral | 36mg/kg (36mg/kg) | LUNGS, THORAX, OR RESPIRATION: ACUTE PULMONARY EDEMA | Clinical Toxicology. Vol. 10, Pg. 327, 1977. |
| mouse | LD50 | intraperitoneal | 75mg/kg (75mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 181, Pg. 68, 1969. | |
| mouse | LD50 | intravenous | 65mg/kg (65mg/kg) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 141, Pg. 83, 1963. |
| mouse | LD50 | oral | 170mg/kg (170mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 118, Pg. 139, 1956. | |
| rabbit | LD50 | intravenous | 33mg/kg (33mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 96, Pg. 209, 1949. | |
| rabbit | LDLo | intraperitoneal | 65mg/kg (65mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 44, Pg. 325, 1932. | |
| rabbit | LDLo | oral | 175mg/kg (175mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 44, Pg. 325, 1932. | |
| rat | LD50 | intraperitoneal | 108mg/kg (108mg/kg) | Pharmacology: International Journal of Experimental and Clinical Pharmacology. Vol. 19, Pg. 182, 1979. | |
| rat | LD50 | oral | 125mg/kg (125mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Federation Proceedings, Federation of American Societies for Experimental Biology. Vol. 7, Pg. 262, 1948. |
| rat | LD50 | subcutaneous | 144mg/kg (144mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 118, Pg. 139, 1956. |