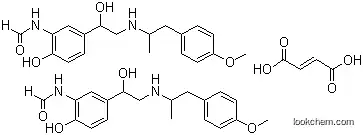
43229-80-7 Usage
Description
Formoterol fumarate is a β2-selective adrenergic
agonist useful in the treatment of bronchial asthma.
It s potency and duration of action a r e reported to
exceed those of salbutamol, as is its ability to inhibit allergic and non-allergic histamine release.
Chemical Properties
Crystalline Solid
Originator
Yarnanouchi (Japan)
Uses
Different sources of media describe the Uses of 43229-80-7 differently. You can refer to the following data:
1. A selective 2-adrenergic receptor agonist. Used as an antiasthmatic
2. antiasthmatic;?2-agonist
3. long-acting β 2-agonist, anti-asthmatic
4. A potent β2-AR agonist
5. Formoterol fumarate is a potent β2-AR agonist.
Brand name
Foradil (Novartis);ATOCK.
Mechanism of action
This is believed to result from formoterol's greater water solubility, allowing it to get to the receptor sites faster, whereas its moderate lipophilicity keeps it in the lungs longer. It is indicated for the long-term maintenance treatment of asthma and for patients with symptoms of nocturnal asthma who require regular treatment with inhaled, short-acting, β2-agonists. It is not indicated for patients whose asthma can be managed by occasional use of inhaled, short-acting, β2-agonists. Formoterol is available only as a powder in a capsule for administration via the aerosolizer. Patients should be cautioned not to take the capsules orally and to keep them in a safe place to avoid accidental oral administration.
Clinical Use
Formoterol has a β-directing N-isopropyl-p-methoxyphenyl group and a unique m-formamide and p-hydroxyphenyl ring, which provides selectivity for β2-receptors. It is resistant to MAO and COMT, making it a long-acting agonist. Formoterol has a more rapid onset as compared to salmeterol while maintaining the same long duration of action.
References
1) Anderson et al. (1993), Formoterol: pharmacology, molecular basis of agonism and mechanism of long duration of a highly potent and selective beta 2-adrenoceptor agonist bronchodilator; Life Sciences, 52 2145
2) Teng et al. (2005), Structure-activity relationship study of novel necroptosis inhibitors; Bioorg. Med. Chem. Lett., 15 5039
3) Noguchi et al. (2015), Effect of beta2-adrenergic agonist on eosinophil adhesion, superoxide anion generation, and degranulation; Allergol. Int., 64 S46
4)Busquets et al. (2004), Anticachectic effects of formoterol: a drug for potential treatment of muscle wasting; Cancer Res., 64 6725
5) O’Neill et al. (2020), Pharmacological targeting of ?2-adrenoceptors is neuroprotective in the LPS inflammatory rat model of Parkinson’s disease; Br. J. Pharmacol., 177 282
Check Digit Verification of cas no
The CAS Registry Mumber 43229-80-7 includes 8 digits separated into 3 groups by hyphens. The first part of the number,starting from the left, has 5 digits, 4,3,2,2 and 9 respectively; the second part has 2 digits, 8 and 0 respectively.
Calculate Digit Verification of CAS Registry Number 43229-80:
(7*4)+(6*3)+(5*2)+(4*2)+(3*9)+(2*8)+(1*0)=107
107 % 10 = 7
So 43229-80-7 is a valid CAS Registry Number.
InChI:InChI=1/C19H24N2O4.C4H4O4/c1-13(9-14-3-6-16(25-2)7-4-14)20-11-19(24)15-5-8-18(23)17(10-15)21-12-22;5-3(6)1-2-4(7)8/h3-8,10,12-13,19-20,23-24H,9,11H2,1-2H3,(H,21,22);1-2H,(H,5,6)(H,7,8)/b;2-1+
43229-80-7Relevant articles and documents
Large-scale synthesis of enantio- and diastereomerically pure (R,R)-formoterol
Hett, Robert,Fang, Qun K.,Gao, Yun,Wald, Stephen A.,Senanayake, Chris H.
, p. 96 - 99 (2013/09/08)
(R,R)-Formoterol (1) is a long-acting, very potent β2-agonist, which is used as a bronchodilator in the therapy of asthma and chronic bronchitis. Highly convergent synthesis of enantio- and diastereomerically pure (R,R)-formoterol fumarate is achieved by a chromatography-free process with an overall yield of 44%. Asymmetric catalytic reduction of bromoketone 4 using as catalyst oxazaborolidine derived from (1R, 2S)-1-amino-2-indanol and resolution of chiral amine 3 are the origins of chirality in this process. Further enrichment of enantio- and diastereomeric purity is accomplished by crystallizations of the isolated intermediates throughout the process to give (R,R)-formoterol (1) as the pure stereoisomer (ee, de >99.5%).