- 55981-09-4Nitazoxanide
- 5598-13-0Phosphorothioic acid,O,O-dimethyl O-(3,5,6-trichloro-2-pyridinyl) ester
- 55984-93-51,4-Benzenedicarbonitrile,2-methyl-
- 55985-32-53,5-Pyridinedicarboxylicacid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 3-methyl5-[2-[methyl(phenylmethyl)amino]ethyl] ester
- 5598-53-8L-Aspartic acid, sodiumsalt (1:2)
- 55985-85-8(2-CARBOXYETHYL)TRIPHENYLPHOSPHONIUM TRIBROMIDE
- 55989-09-8Pethidine HCl
- 559-91-12,2,3,3,4,4,4-Heptafluoro-1-phenyl-butan-1-one
- 55-99-21,4-bis(methylsulfonylsulfanyl)butane
- 55994-13-3Benzenesulfonic acid,3-[2-(4-amino-3-methylphenyl)diazenyl]-
Hot Products
- 920-37-62-Chloroacrylonitrile
- 95233-18-4Atovaquone
- 20123-80-2Calcium dobesilate
- 7773-06-0Sulfamic acid, ammoniumsalt (1:1)
- 387-45-1Benzaldehyde, 2-chloro-6-fluoro-
- 98-09-9Benzenesulfonyl chloride
- 13811-71-7(-)-Diethyl D-tartrate
- 84-69-51,2-Benzenedicarboxylicacid, 1,2-bis(2-methylpropyl) ester
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin

|
Basic Information |

|
Post buying leads |

|
Suppliers |

|
Cas Database |

| Name |
Busulfan |
EINECS | 200-250-2 |
| CAS No. | 55-98-1 | Density | 1.35 g/cm3 |
| PSA | 103.50000 | LogP | 1.88060 |
| Solubility | Decomposes in water | Melting Point |
114-118 °C |
| Formula | C6H14O6S2 | Boiling Point | 464 °C at 760 mmHg |
| Molecular Weight | 246.306 | Flash Point | 234.4 °C |
| Transport Information | UN 2811 6.1/PG 1 | Appearance | white crystalline solid |
| Safety | 53-36/37/39-45-28A | Risk Codes | 45-26/27/28-63-46-36/37/38-23/24/25 |
| Molecular Structure |
|
Hazard Symbols |
 T+, T+, T T
|
| Synonyms |
1,4-Butanediol,dimethanesulfonate (8CI,9CI);Methanesulfonic acid, tetramethylene ester (6CI);1,4-Butanediol dimesylate;Busilvex;Busulphan;Citosulfan;GT 2041;GT 41;Glyzophrol;Leucosulfan;Mielevcin;Mielucin;Mileran;Misulban;Myeloleukon;Mylecytan;Sulfabutin;Tetramethylene bis[methanesulfonate]; |
Article Data | 23 |
Busulfan Synthetic route

| Conditions | Yield |
|---|---|
| With triethylamine at 5 - 40℃; for 4h; Temperature; | 91% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 10 - 20℃; for 24h; | 86% |
| With triethylamine In dichloromethane at 0 - 20℃; for 4h; | 73.52% |
| With pyridine at 25℃; for 2h; | 55% |
- 109-99-9
tetrahydrofuran

- 41138-92-5
methanesulfonyl bromide

A
- 110-52-1
1,4-dibromo-butane

B
- 7239-41-0
4,4'-dibromo-di-n-butyl ether

C
- 55-98-1
busulfan

D
- 40671-33-8
1-bromo-4-methanesulfonyloxy-butane

| Conditions | Yield |
|---|---|
| zinc(II) chloride at 75℃; for 5h; |


| Conditions | Yield |
|---|---|
| In acetonitrile for 5h; Heating; |

| Conditions | Yield |
|---|---|
| With tin(IV) chloride at 80 - 100℃; |
- 96042-30-7
4-(dicyanomethylene)-2-methyl-6-(p-dimethylaminostyryl)-4H-pyran

- 124-63-0
methanesulfonyl chloride

- 55-98-1
busulfan

| Conditions | Yield |
|---|---|
| With triethylamine In water; ethyl acetate |
- 55-98-1
busulfan

- 1182838-48-7
1,10-N,N'-bis-[cyclomaltoheptaosyl-6A-deoxy-6A-ureido]-4,7,13,16-tetraoxa-1,10-diazacyclooctadecane

- 1182838-51-2
C6H14O6S2*C98H164N4O74

| Conditions | Yield |
|---|---|
| In water; dimethyl sulfoxide at 20℃; for 18h; Inert atmosphere; | 100% |
- 55-98-1
busulfan

- 1182838-47-6
1,10-N,N'-bis-(β-D-ureidocellobiosyl)-4,7,13,16-tetraoxa-1,10-diazacyclooctadecane

- 1182838-49-8
C6H14O6S2*C38H68N4O26

| Conditions | Yield |
|---|---|
| In water; dimethyl sulfoxide at 20℃; for 18h; Inert atmosphere; | 100% |
- 55-98-1
busulfan

- 138566-17-3
2-(benzhydrylideneamino)-1-(10,10-dimethyl-3,3-dioxo-3λ6-thia-4-aza-tricyclo[5.2.1.01.5]dec-4-yl)ethanone

| Conditions | Yield |
|---|---|
| Stage #1: 2-(benzhydrylideneamino)-1-(10,10-dimethyl-3,3-dioxo-3λ6-thia-4-aza-tricyclo[5.2.1.01.5]dec-4-yl)ethanone With lithium hexamethyldisilazane In tetrahydrofuran at -78℃; for 0.333333h; Inert atmosphere; Stage #2: busulfan In tetrahydrofuran at -78℃; for 3.16667h; | 83% |
Busulfan Chemical Properties
Structure of Busulfan (CAS NO.55-98-1):
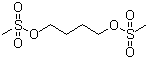
IUPAC Name: 4-methylsulfonyloxybutyl methanesulfonate
Molecular Formula: C6H14O6S2
Molar mass: 246.30176 g/mol
H bond acceptors: 6
H bond donors: 0
Freely Rotating Bonds: 7
Polar Surface Area: 103.5Å2
Index of Refraction: 1.47
Molar Refractivity: 50.94 cm3
Molar Volume: 182.3 cm3
Surface Tension: 46.6 dyne/cm
Density: 1.35 g/cm3
Flash Point: 234.4 °C
Enthalpy of Vaporization: 69.75 kJ/mol
Boiling Point: 464 °C at 760 mmHg
Vapour Pressure: 2.41E-08 mmHg at 25°C
Appearance: White Crystalline Solid
Melting point: 114-118°C
Solubility: Solubility in acetone at 25 °C for 2.4g/100ml, in ethanol solubility for 0.1g/100ml, almost no smell.
Water solubility:Decomposes
Busulfan History
Busulfan was the mainstay of the chemotherapeutic treatment of Chronic Myeloid Leukemia (CML) until it was displaced by the new gold standard, Imatinib, though it is still in use to a degree as a result of the drug's relative inexpense.
Busulfan Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| bird - wild | LD50 | oral | 56200ug/kg (56.2mg/kg) | Archives of Environmental Contamination and Toxicology. Vol. 12, Pg. 355, 1983. | |
| dog | LDLo | intravenous | 8mg/kg (8mg/kg) | BLOOD: LEUKOPENIA BLOOD: AGRANULOCYTOSIS BLOOD: OTHER CHANGES | Cancer Chemotherapy Reports, Part 2. Vol. 2, Pg. 203, 1965. |
| man | TDLo | oral | 8mg/kg/2D-I (8mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Lancet. Vol. 2, Pg. 1463, 1984. |
| monkey | LDLo | intravenous | 8mg/kg (8mg/kg) | BLOOD: LEUKOPENIA BLOOD: OTHER CHANGES BLOOD: AGRANULOCYTOSIS | Cancer Chemotherapy Reports, Part 2. Vol. 2, Pg. 203, 1965. |
| mouse | LD50 | intraperitoneal | 86mg/kg (86mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Kiso to Rinsho. Clinical Report. Vol. 5, Pg. 1894, 1971. |
| mouse | LD50 | oral | 110mg/kg (110mg/kg) | GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" SKIN AND APPENDAGES (SKIN): HAIR: OTHER | Kiso to Rinsho. Clinical Report. Vol. 5, Pg. 1894, 1971. |
| mouse | LD50 | subcutaneous | 63mg/kg (63mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Kiso to Rinsho. Clinical Report. Vol. 5, Pg. 1894, 1971. |
| mouse | LD50 | unreported | 46mg/kg (46mg/kg) | British Journal of Cancer. Vol. 6, Pg. 160, 1952. | |
| rat | LD50 | intraperitoneal | 18mg/kg (18mg/kg) | GASTROINTESTINAL: OTHER CHANGES BLOOD: CHANGES IN BONE MARROW NOT INCLUDED ABOVE | Biochemical Pharmacology. Vol. 1, Pg. 39, 1958. |
| rat | LD50 | intravenous | 1800ug/kg (1.8mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 20, Pg. 1467, 1970. | |
| rat | LD50 | subcutaneous | 22mg/kg (22mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Kiso to Rinsho. Clinical Report. Vol. 5, Pg. 1894, 1971. |
| rat | LD50 | unreported | 10mg/kg (10mg/kg) | Chemical Abstracts. Vol. 55, Pg. 10679a, 1961. | |
| rat | LDLo | oral | 15mg/kg (15mg/kg) | Khimiya i Meditsina. Vol. 13, Pg. 34, 1960. | |
| women | TDLo | oral | 80mg/kg/8Y (80mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE VASCULAR: REGIONAL OR GENERAL ARTERIOLAR OR VENOUS DILATION GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS | JAMA, Journal of the American Medical Association. Vol. 238, Pg. 1951, 1977. |
Busulfan Consensus Reports
NTP 10th Report on Carcinogens. IARC Cancer Review: Group 1 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 (1987),p. 137.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 4 (1974),p. 247.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Human Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 4 (1974),p. 247.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . EPA Genetic Toxicology Program.
Busulfan Safety Profile
Confirmed carcinogen producing leukemia, kidney, and uterine tumors. Experimental neoplastigenic and tumorigenic data. Poison by ingestion, subcutaneous, intraperitoneal, intravenous, and possibly other routes. Ingestion by pregnant women can cause cancer of the reproductive system of the fetus including the uterus. Human teratogenic effects by ingestion and possibly other routes include developmental abnormalities of the eye, ear, craniofacial area including the nose and tongue, gastrointestinal system, endocrine system, urogenital system, and other unspecified areas. Other human reproductive effects by ingestion and possibly other routes include: impotence, changes in the uterus, cervix, and vagina, and menstrual-cycle disorders. Experimental reproductive effects. Human systemic effects by ingestion: general arteriolar or venous dilation of the eye, changes in structure or function of salivary glands. When heated to decomposition it emits toxic fumes of SOx. See also Hazard SULFONATES.
Codes: T+ ,T
,T
Risk Statements:
23: Toxic by inhalation
24: Toxic in contact with skin
25: Toxic if swallowed
26: Very Toxic by inhalation
27: Very Toxic in contact with skin
28: Very Toxic if swallowed
36: Irritating to the eyes
37: Irritating to the respiratory system
38: Irritating to the skin
45: May cause cancer
46: May cause heritable genetic damage
63: Possible risk of harm to the unborn child
Safety Statements:
28: After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer)
36: Wear suitable protective clothing
37: Wear suitable gloves
39: Wear eye/face protection
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
63: In case of accident by inhalation, remove casualty to fresh air and keep at rest
RIDADR: UN 2811 6.1/PG 1
WGK Germany: 3
RTECS: EK1750000
HazardClass: 6.1(b)
PackingGroup: III
Busulfan Specification
Busulfan , with CAS number of 55-98-1, can be called 1,4-Bis(methyl sulfonoxy)butane;busulphan ; 1,4-dimethanesulfonoxybutane ; 1,4-Butanediol dimethyl sulfonate; busulfex ; tetramethylene bis(methanesulfonate) . It is a white crystalline solid, Busulfan (CAS NO.55-98-1) can be used as alkylating agent with antileukemic activity. It can be used for antineoplastic.

