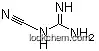
461-58-5Relevant articles and documents
Nitrosation of Cyanamide: Preparation and Properties of the Elusive E- and Z-N'-Cyanodiazohydroxides
Guethner, Thomas,Huber, Evi,Sans, Juergen,Thalhammer, Franz
supporting information, (2020/04/29)
Nitrosation of cyanamide leads to unstable E/Z-cyanodiazohydroxides that easily deprotonate to E/Z-cyanodiazotates. Pursuing observations of E. Drechsel 145 years ago, the structure and reactivity of those products was determined, mainly in aqueous solution. Depending on the pH, three different thermal decomposition pathways give either N2O + HCN or N2 + HNCO. They were evaluated experimentally and by quantum mechanical calculations.
Novel method for synthesis of sulphaguanidine
Singh, Vikram,Kaushik
experimental part, p. 645 - 648 (2012/05/04)
The microwave-enhanced synthesis of sulphaguanidine is achieved rapidly and in good yield via the step-wise reaction of cyanamide with sulphonamide in the presence of acidic alumina or montmorillonite clays (KSF and K10).
Formation of pyrimidin-2-ylcyanamide and 2-aminopyrimidine in the reaction of aniline derivatives with cyanamide and dimethylamino-1-pyridyl-2-propenone
Koroleva,Ignatovich, Zh.V.,Ignatovich,Gusak
scheme or table, p. 1222 - 1226 (2011/12/01)
Substituted o- and p-nitroanilines and m-benzylaminoanilines in the reaction with cyanamide failed to yield the corresponding arylguanidines, and in the presence of 3-dimethylamino-1-(3-pyridyl)-2-propen-1-one formed 4-pyridyl-substituted pyrimidin-2-ylcyanamides and 2-amino-pyrimidines.
Reaction of N-(carbamimidoyl)thiourea with 1-benzoyl-2-phenylacetylene
Glotova,Dvorko,Albanov,Protsuk
, p. 121 - 125 (2007/10/03)
1-Benzoyl-2-phenylacetylene reacted with N-(carbamimidoyl)thiourea in glacial acetic acid saturated with 20% HBr to give (4,6-diphenyl-2H-1,3-thazin- 2-ylidene)guanidine hydrobromide. The reaction of the same compounds in anhydrous ethanol in the presence
2-substituted-4-nitrogen heterocycles as modulators of chemokine receptor activity
-
, (2008/06/13)
The present application describes modulators of CCR3 of formula (I): or pharmaceutically acceptable salt forms thereof, useful for the prevention of asthma and other allergic diseases.
CRF receptor antagonists and methods relating thereto
-
, (2008/06/13)
CRF receptor antagonists are disclosed which have utility in the treatment of a variety of disorders, including the treatment of disorders manifesting hypersecretion of CRF in a warm-blooded animals, such as stroke. The CRF receptor antagonists of this invention have the following structure: including stereoisomers, prodrugs and pharmaceutically acceptable salts thereof, wherein m, R, R1, R2, A, X, Y and Z are as defined herein. Compositions containing a CRF receptor antagonist in combination with a pharmaceutically acceptable carrier are also disclosed, as well as methods for use of the same.
Bis(trimesitylgermylcarbodiimido)germylene, trimesitylgermylcyanamide and trimesitylgermylcarbodiimide
Rivière-Baudet, Monique,Dahrouch, Mohamed,Gornitzka, Heinz
, p. 153 - 157 (2007/10/03)
Bis(trimesitylgermylcarbodiimido)germylene (1), isolated as a white precipitate from the reaction of lithium trimesitylgermylcyanamide with dichlorogermylene, is stable at room temperature in the absence of water and oxygen. The germylene structure is pre
Kinetics of the reaction of β-methoxy-α-nitrostilbene with cyanamide in 50% DMSO-50% water. Failure to detect the S(N)V intermediate
Bernasconi, Claude F.,Leyes, Aquiles E.,Rappoport, Zvi
, p. 2897 - 2902 (2007/10/03)
A kinetic study of the reaction of β-methoxy-α-nitrostilbene (1-OMe) with cyanamide (CNA) over a pH range from 8.5 to 12.4 shows that it is the anion (CNA-, pK(a) = 11.38) rather than the neutral amine that is the reactive species. Attempts at monitoring the reaction with the neutral CNA at low pH were unsuccessful because of competing hydrolysis. It is shown that the nucleophilic reactivity of CNA is abnormally low, probably because of a resonance effect, and that the reactivity of CNA- is high, higher than that of strongly basic oxyanion because of relatively weak solvation. The high reactivity of both 1-OMe and CNA- appeared to constitute favorable conditions conducive to the detection of the S(N)V intermediate, as has been the case in the reactions of 1-OMe with thiolate ions, alkoxide ions, and some amines. However, no intermediate was observed. Reasons for this failure are discussed.
Formation of Oxygen-bridged Heterocycles in the Hantzsch Synthesis with 4-(2-Hydroxyphenyl)but-3-en-2-one
Svetlik, Jan,Turecek, Frantisek,Hanus, Vladimir
, p. 2053 - 2058 (2007/10/02)
Condensations of 4-(2-hydroxyphenyl)but-3-en-2-one with methyl acetoacetate, pentane-2,4-dione, dimedone, and methyl cyanoacetate under the conditions of the Hantzsch synthesis lead to 9-methyl-8-oxa-10-azatricyclo2,7>trideca-2,4,6,11-tetraene derivatives and related heterocycles.An analogous condensation with 2-aminopropene-1,1,3-triscarbonitrile yields stereoselectively 11-dicyanomethylene-9-methyl-8-oxa-10-azatricyclo2,7>trideca-2,4,6-triene-12α-carbonitrile.Under suitable reaction conditions, the condensation of 4-(2-hydroxyphenyl)but-3-en-2-one with cyanamide affords various products with the 9-methyl-8-oxa-10,12-diazatricyclo2,7>trideca-2,4,6,11-tetraene skeleton.Condensation products of salicylaldehyde with acetone, 2-aminopropene-1,1,3-triscarbonitrile, and 3-aminocrotononitrile have been identified.
Heterocyclic amidino substituted ureas and their pharmaceutical uses
-
, (2008/06/13)
This invention relates to methods for the prophylactic and curative treatment of gastrointestinal and cardiovascular disorders and parasitic infections in humans and animals, using a class of hetrocyclic amidino substituted urea and thiourea compounds, a novel class of heterocyclic amidino substituted urea and thiourea compounds and pharmaceutical compositions and animal feed additives including the same.