- Ciprofloxacin
- Ciprofloxacin Hydrochloride
- Ciprofloxacin hydrochloride
- Ciprofloxacin hydrochloride hydrate
- Ciprofloxacin lactate
- 857220-13-4C-(6-BROMO-PYRIDIN-3-YL)-METHYLAMINE HYDROCHLORIDE
- 85722-07-2Titanium,bis[(1,2,3,4,5-h)-1-butyl-2,4-cyclopentadien-1-yl]difluoro-
- 85-72-3Benzoic acid,2-[[(3-methylphenyl)amino]carbonyl]-
- 85723-72-41-Piperidinepropanamine,2,6-dimethyl-
- 857272-02-72H-1,4-Benzoxazin-3(4H)-one,6-(chloromethyl)-4-(3-methoxypropyl)-
- 857278-37-61-Piperidinecarboxylicacid, 3-hydroxy-4-(4-hydroxyphenyl)-, phenylmethylester, (3R,4R)-
- 857283-57-94-Isoxazolemethanamine,N,5-dimethyl-3-phenyl-
- 857283-58-0Imidazo[1,2-a]pyridine-3-methanamine,N,2-dimethyl-
- 857283-59-11H-Pyrrole,1-(3-isocyanatophenyl)-
- 857283-60-41H-Pyrrole,1-(4-isocyanatophenyl)-
Hot Products
- 154598-52-4Efavirenz
- 90-05-1Guaiacol
- 54-85-3Isoniazid
- 147-71-7Butanedioic acid, 2,3-dihydroxy-, (2S,3S)-
- 181289-15-63-Carbamoymethyl-5-methylhexanoic acid
- 544-17-2Calcium formate
- 99-93-44'-Hydroxyacetophenone
- 124752-23-4tert-Butyl (4R-cis)-6-formaldehydel-2,2-dimethyl-1,3-dioxane-4-acetate
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin

|
Basic Information |

|
Post buying leads |

|
Suppliers |

|
Cas Database |

| Name |
Ciprofloxacin |
EINECS | 617-751-0 |
| CAS No. | 85721-33-1 | Density | 1.461 g/cm3 |
| PSA | 74.57000 | LogP | 1.97710 |
| Solubility | 86mg/L(25 oC) | Melting Point |
255-257 °C |
| Formula | C17H18F N3 O3 | Boiling Point | 581.774 °C at 760 mmHg |
| Molecular Weight | 331.347 | Flash Point | 305.646 °C |
| Transport Information | N/A | Appearance | White powder |
| Safety | 26-36 | Risk Codes | 36/37/38 |
| Molecular Structure |
|
Hazard Symbols |
 Xi Xi
|
| Synonyms |
1-Cyclopropyl-6-fluoro-1,4-dihydro-7-(1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid;1-Cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylicacid;BAY-q 3939;Catex;Ciflafin;Ciprine;Cipro IV;Ciprobay 100;Ciprofloxacillin;Ciprofloxacin;Ciprofloxacine;Ciprolet;Ciprolet DS;Cipromed;Cipropol;Ciprovet;Ciproxim;Ciproxina;Cunesin;Cyclofloxacin;Euciprin;Oftacifox;Procip;Quinox XR; |
Article Data | 62 |
Ciprofloxacin Synthetic route
- 93594-48-0
7-[4-tert-butoxycarbonyl-piperazin-1-yl]-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-quinoline-3-carboxylic acid

- 85721-33-1
ciprofloxacin

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane at 0 - 20℃; for 2h; | 98.7% |

| Conditions | Yield |
|---|---|
| With 1-butyl-3-methylimidazolium hydroxide at 70℃; for 5h; Reagent/catalyst; Temperature; | 96.2% |
- 93107-08-5
ciprofloxacin hydrochloride

- 85721-33-1
ciprofloxacin

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water | 96% |
| With sodium hydrogencarbonate In water | 95% |
| With ammonium hydroxide In water at 30 - 55℃; for 4.41667h; pH=6.8; Temperature; Reagent/catalyst; pH-value; | 82.52% |
- 110-85-0
piperazine

- 86393-33-1
7-chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-3-quinoline carboxylic acid

- 85721-33-1
ciprofloxacin

| Conditions | Yield |
|---|---|
| With nano iron oxide on ZrO2 coated sulfonic acid In water for 0.366667h; Reflux; | 94% |
| at 150℃; Microwave irradiation; | 90% |
| With aluminum tri-bromide In ethanol at 75℃; for 4h; Reagent/catalyst; Solvent; Temperature; | 89.7% |

- 110-85-0
piperazine

- 85721-33-1
ciprofloxacin

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In i-Amyl alcohol at 140℃; for 8h; Reagent/catalyst; Temperature; | 91.2% |
- 110-85-0
piperazine

- 93107-30-3
1-cyclopropyl-6,7-difluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid

- 85721-33-1
ciprofloxacin

| Conditions | Yield |
|---|---|
| In pyridine for 18h; Heating; | 89% |
| In water | |
| In dimethyl sulfoxide Heating; | 960 mg |

- 86393-33-1
7-chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-3-quinoline carboxylic acid

- 85721-33-1
ciprofloxacin

| Conditions | Yield |
|---|---|
| 88% |
- 110-85-0
piperazine

- 86393-33-1
7-chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-3-quinoline carboxylic acid

A
- 85721-33-1
ciprofloxacin

| Conditions | Yield |
|---|---|
| In water for 15h; Heating; | A 86% B n/a |
| In water at 150℃; for 5h; | A 65% B 10% |
| In dimethylsulfoxide-d6 at 120 - 130℃; | A 87 % Spectr. B 13 % Spectr. |

- 85721-33-1
ciprofloxacin

| Conditions | Yield |
|---|---|
| With adsorption onto charcoal; trifluoroacetic acid 2.) dichloromethane; | 57% |
| With adsorption onto charcoal; trifluoroacetic acid In dichloromethane Product distribution; solid/solution phase synthesis to eliminate impurities; | |
| With water; pyrographite; trifluoroacetic acid In dichloromethane at 20℃; for 0.25h; Hydrolysis; |
- 110-85-0
piperazine

- 98349-24-7
ethyl 2,4,5-trifluorobenzoylacetate

- 4637-24-5
N,N-dimethyl-formamide dimethyl acetal

- 765-30-0
Cyclopropylamine

- 85721-33-1
ciprofloxacin

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction; |
Ciprofloxacin Chemical Properties
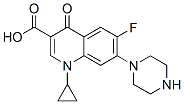
IUPAC Name: 1-cyclopropyl-6-fluoro-4-oxo-7-piperazin-4-ium-1-ylquinoline-3-carboxylate
Molecular Formula: C17H18FN3O3
Molecular Weight: 331.341523 g/mol
Appearance: White Powder
Melting Point: 255-257 °C
storage temp.: Store at 0-5 °C
Merck: 13,2337
Density: 1.461 g/cm3
Flash Point: 305.6 °C
Index of Refraction: 1.655
Surface Tension: 67.4 dyne/cm
Enthalpy of Vaporization: 91.5 kJ/mol
Boiling Point: 581.8 °C at 760 mmHg
Vapour Pressure: 2.24E-14 mmHg at 25 °C
Synonyms of Ciprofloxacin (CAS NO.85721-33-1): 1-cyclopropyl-6-fluoro-4-oxo-7-(1-piperazinyl)-1,4-dihydro-3-quinolinecarbox ; ciloxan; ciprobid ; ciproiv ; euciprin ; ciprobay ; ciprofloxacin ; 1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid
Categories: Active Pharmaceutical Ingredients;Intermediates & Fine Chemicals;Pharmaceuticals
Ciprofloxacin History
Ciprofloxacin (CAS NO.85721-33-1) was first introduced as the broad-spectrum oral fluoroquinolone in 1987 by Bayer. In 1991 the intravenous formulation was introduced. Ciprofloxacin (85721-33-1) was first patented Jan 21, 1986, in Germany, and was later approved by the U.S. Food and Drug Administration on October 22, 1987.
Ciprofloxacin Uses
Ciprofloxacin (CAS NO.85721-33-1) is a third-generation quinolone antibiotics used for the treatment of respiratory tract infections, urinary tract, ENT, skin and soft tissue, gastrointestinal tract infections. It is also used as fluorinated quinolone antibacterial.
Ciprofloxacin Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| man | LDLo | oral | 5714ug/kg/2D- (5.714mg/kg) | BEHAVIORAL: COMA LIVER: "JAUNDICE, OTHER OR UNCLASSIFIED" LIVER: LIVER FUNCTION TESTS IMPAIRED | Lancet. Vol. 343, Pg. 738, 1994. |
| man | TDLo | oral | 21mg/kg/3D-I (21mg/kg) | KIDNEY, URETER, AND BLADDER: URINE VOLUME DECREASED KIDNEY, URETER, AND BLADDER: PROTEINURIS KIDNEY, URETER, AND BLADDER: HEMATURIA | Annals of Pharmacotherpy. Vol. 29, Pg. 84, 1995. |
| man | TDLo | oral | 21429ug/kg/3D (21.429mg/kg) | BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" BEHAVIORAL: TOXIC PSYCHOSIS | Annals of Pharmacotherpy. Vol. 26, Pg. 930, 1992. |
| man | TDLo | oral | 129mg/kg/6D-I (129mg/kg) | BEHAVIORAL: REGIDITY | Journal of Clinical Psychiatry. Vol. 54, Pg. 115, 1993. |
| man | TDLo | oral | 200mg/kg (200mg/kg) | BEHAVIORAL: TREMOR GASTROINTESTINAL: NAUSEA OR VOMITING GASTROINTESTINAL: OTHER CHANGES | Annals of Pharmacotherpy. Vol. 28, Pg. 805, 1994. |
| mouse | LD50 | intraperitoneal | 1165mg/kg (1165mg/kg) | Ensho. Japanese Journal of Inflammation. Vol. 11, Pg. 343, 1991. | |
| mouse | LD50 | intravenous | 122mg/kg (122mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Korean Journal of Toxicology. Vol. 8, Pg. 161, 1993. |
| mouse | LD50 | oral | 5gm/kg (5000mg/kg) | Journal of Medicinal Chemistry. Vol. 33, Pg. 1344, 1990. | |
| mouse | LD50 | subcutaneous | > 1gm/kg (1000mg/kg) | Zhongguo Yaoxue Zazhi. Chinese Pharmacuetical Journal. Vol. 27, Pg. 297, 1992. | |
| rat | LD50 | intravenous | 207mg/kg (207mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Korean Journal of Toxicology. Vol. 8, Pg. 161, 1993. |
| rat | LD50 | oral | > 2gm/kg (2000mg/kg) | Zhongguo Yaoxue Zazhi. Chinese Pharmacuetical Journal. Vol. 27, Pg. 297, 1992. | |
| rat | LD50 | subcutaneous | > 1gm/kg (1000mg/kg) | Zhongguo Yaoxue Zazhi. Chinese Pharmacuetical Journal. Vol. 27, Pg. 297, 1992. | |
| women | TDLo | multiple routes | 40mg/kg/6D-I (40mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, ALLERGIC: AFTER SYSTEMIC EXPOSURE" SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | Acta Clinica Belgica. Vol. 49, Pg. 173, 1994. |
| women | TDLo | oral | 10mg/kg (10mg/kg) | BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" GASTROINTESTINAL: NAUSEA OR VOMITING | Allergy. Vol. 50(Suppl, |
| women | TDLo | oral | 40mg/kg/2D-I (40mg/kg) | BEHAVIORAL: HEADACHE BLOOD: EOSINOPHILIA | American Journal of Medicine. Vol. 87, Pg. 589, 1989. |
| women | TDLo | oral | 135mg/kg/3D-I (135mg/kg) | KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" | Archives of Internal Medicine. Vol. 153, Pg. 1258, 1993. |
| women | TDLo | oral | 280mg/kg/2W-I (280mg/kg) | KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" KIDNEY, URETER, AND BLADDER: INTERSTITIAL NEPHRITIS SKIN AND APPENDAGES (SKIN): "DERMATITIS, ALLERGIC: AFTER SYSTEMIC EXPOSURE" | American Journal of Kidney Diseases. Vol. 22, Pg. 598, 1993. |
Ciprofloxacin Safety Profile
Safety Information of Ciprofloxacin (CAS NO.85721-33-1):
Hazard Codes: Xi 
Risk Statements: 36/37/38
36/37/38:Irritating to eyes, respiratory system and skin
Safety Statements: 26-36
26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36:Wear suitable protective clothing
WGK Germany: 2
RTECS: VB1993800

