
Organic Letters p. 6000 - 6003 (2012)
Update date:2022-08-15
Topics:
 Lauer, Matthew G.
Lauer, Matthew G.
 Henderson, William H.
Henderson, William H.
 Awad, Amneh
Awad, Amneh
 Stambuli, James P.
Stambuli, James P.
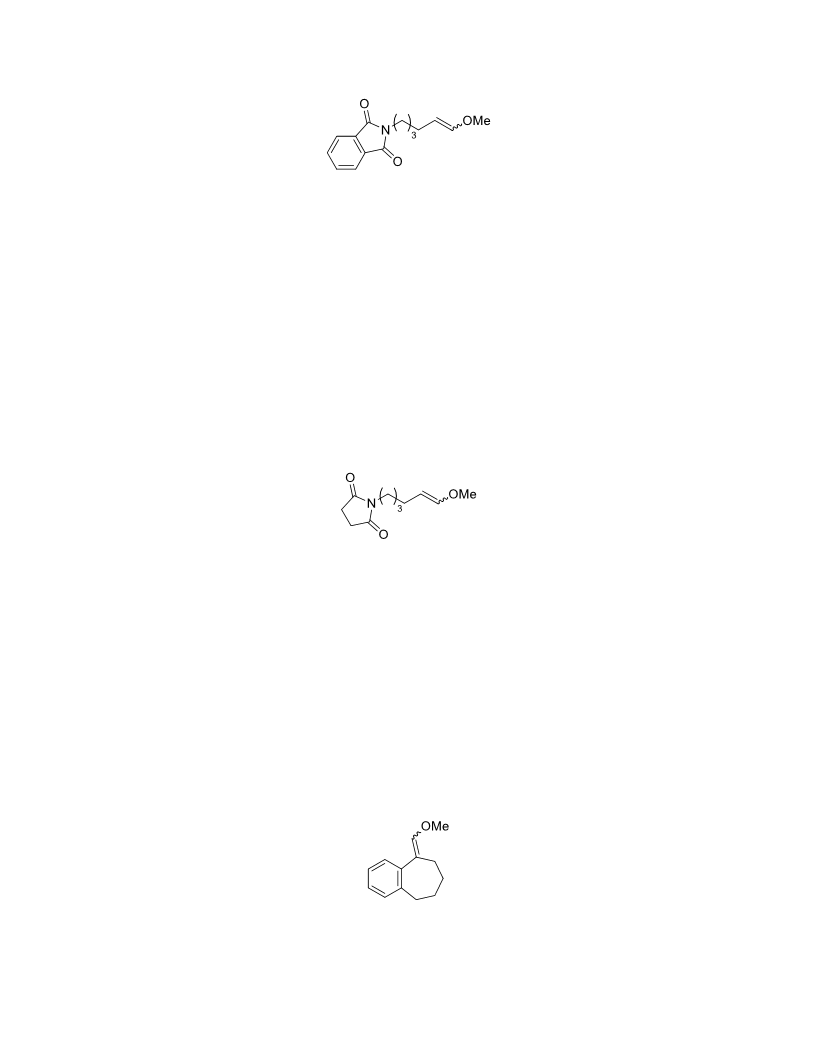
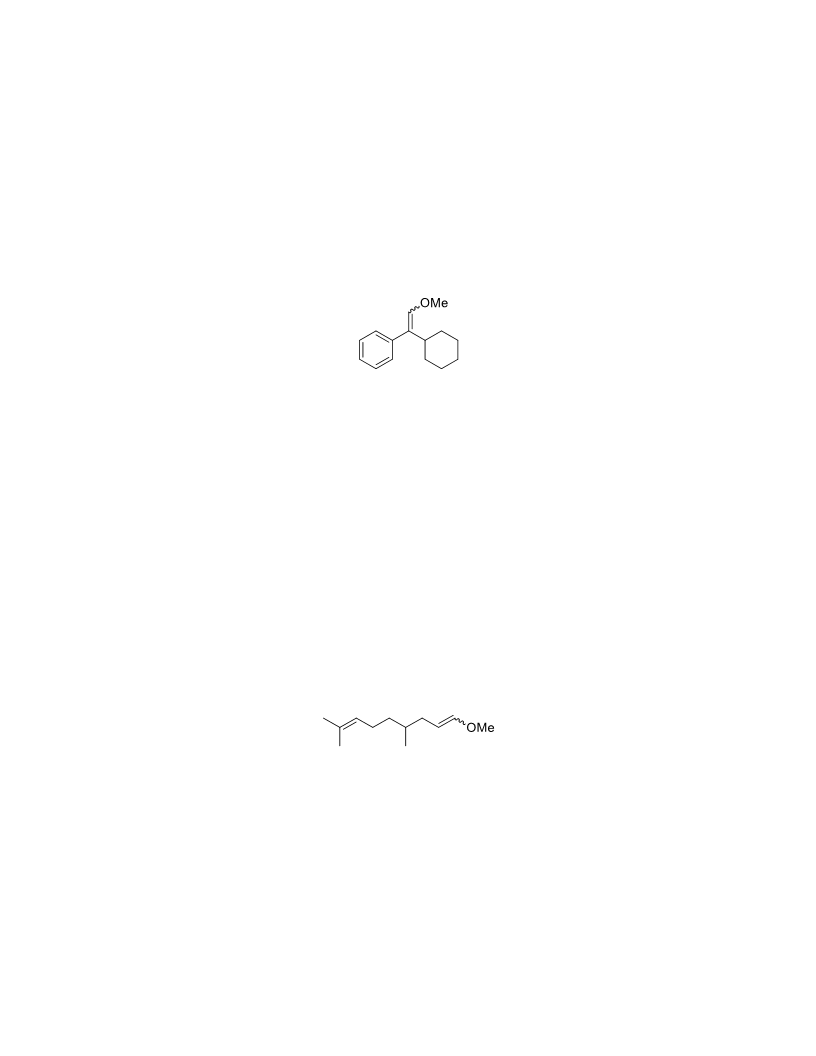
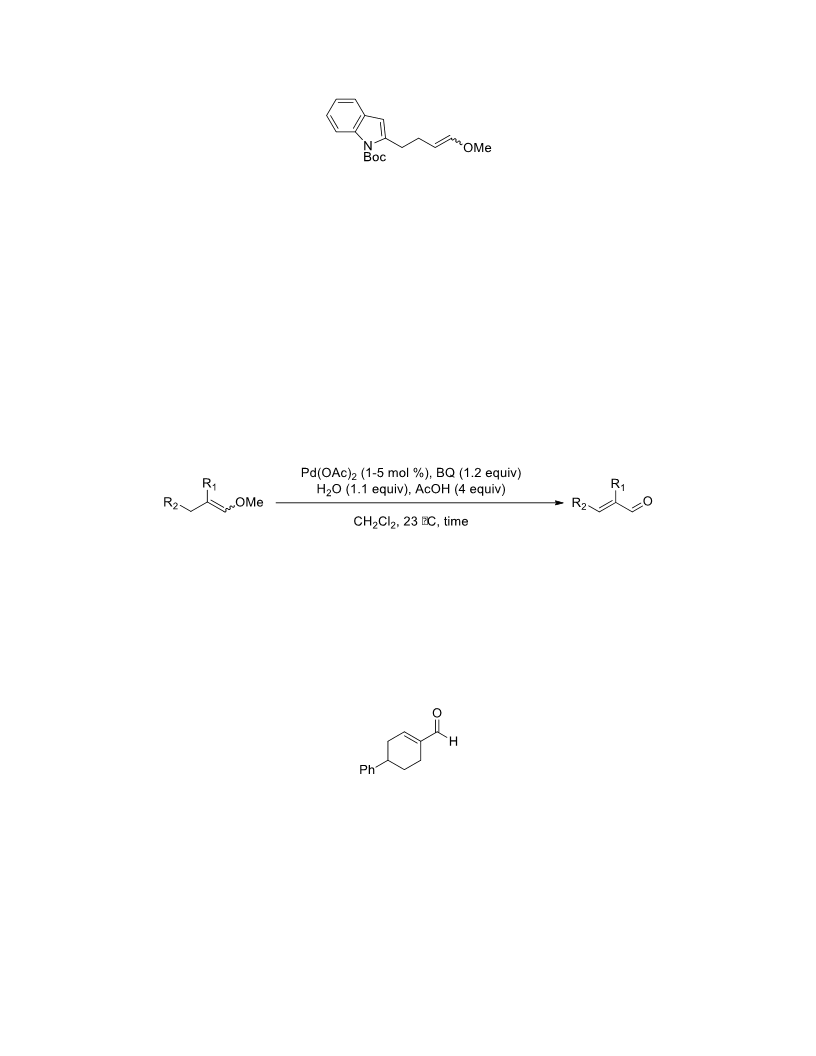
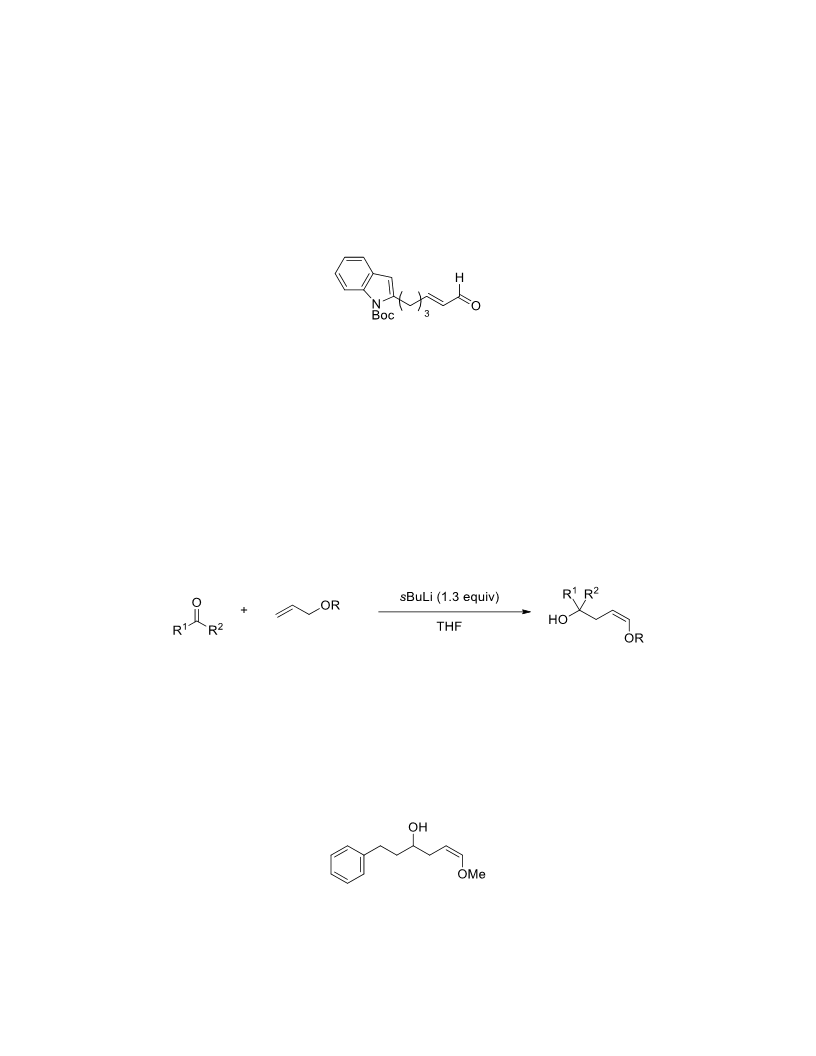
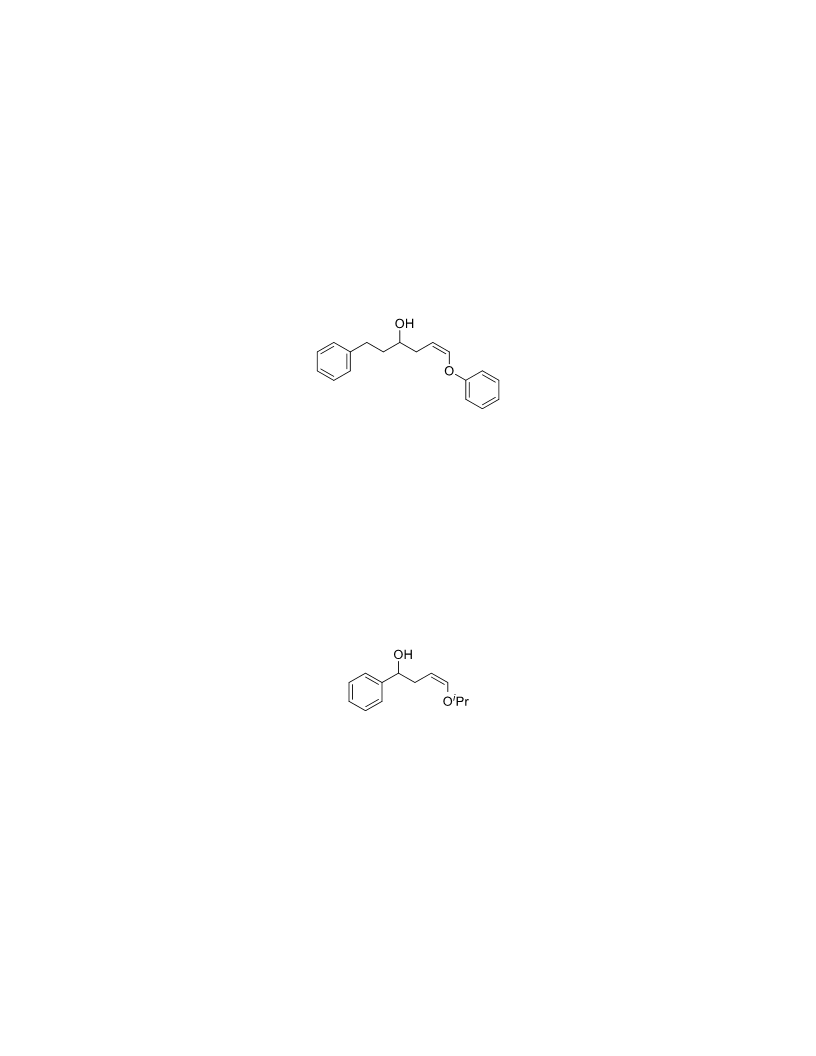
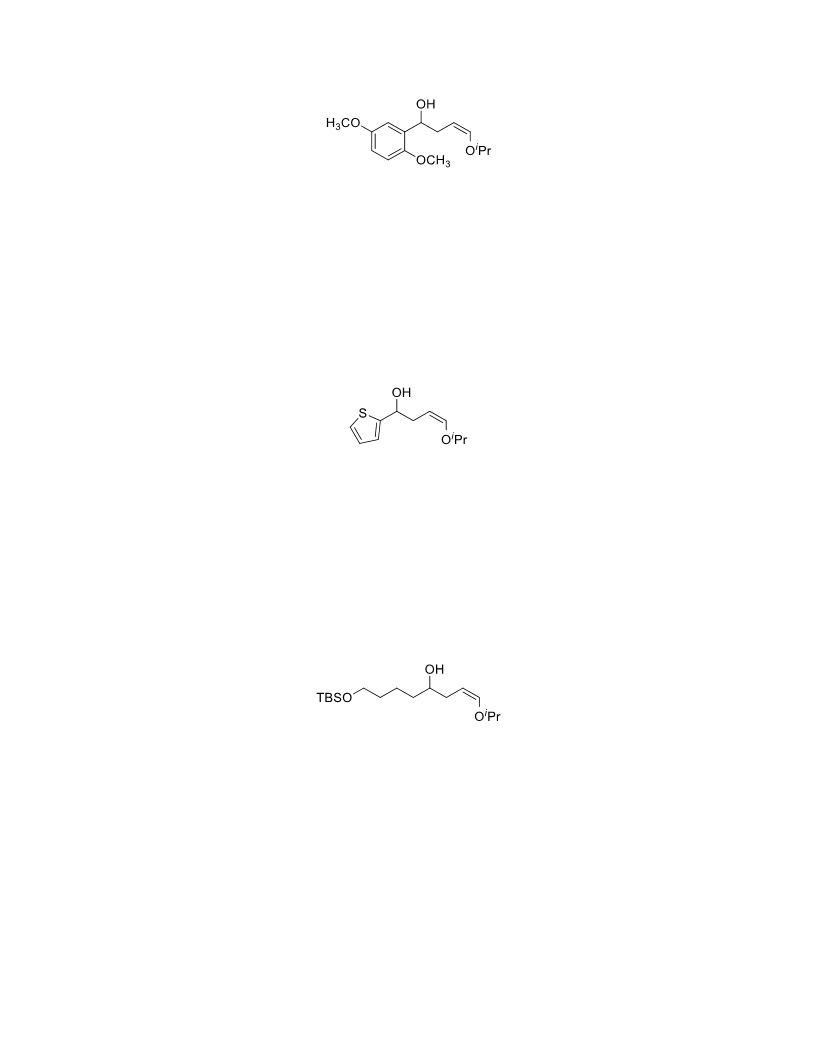
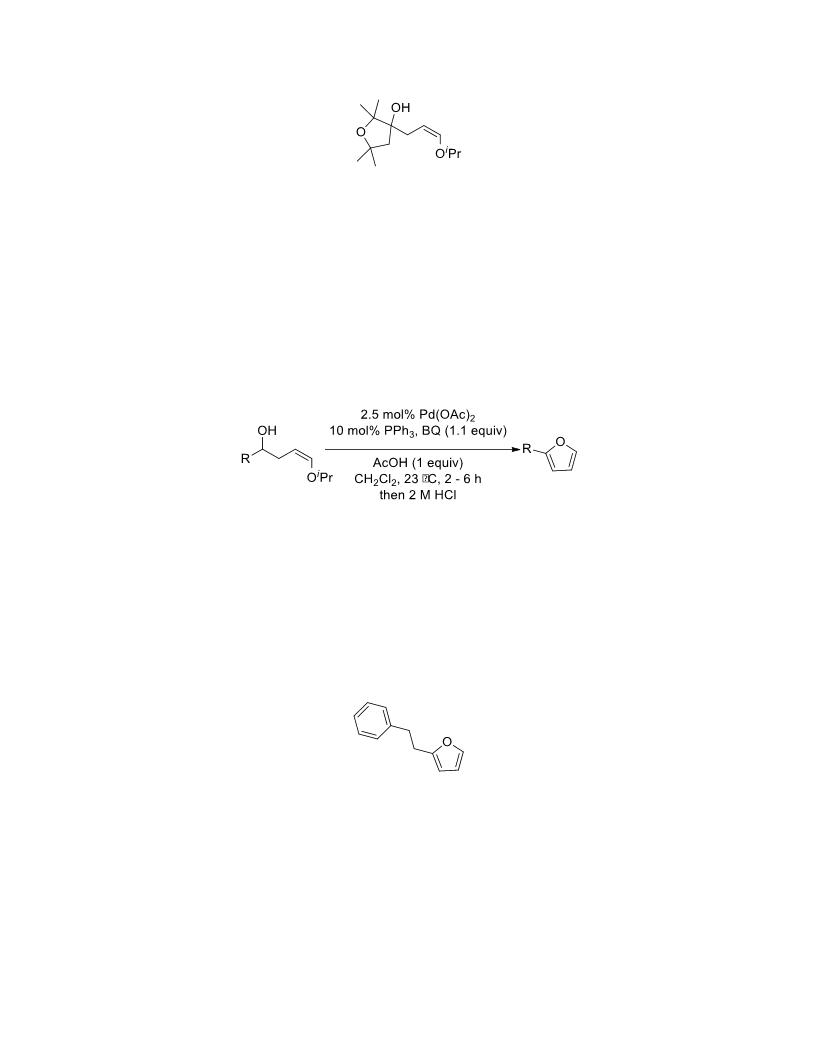
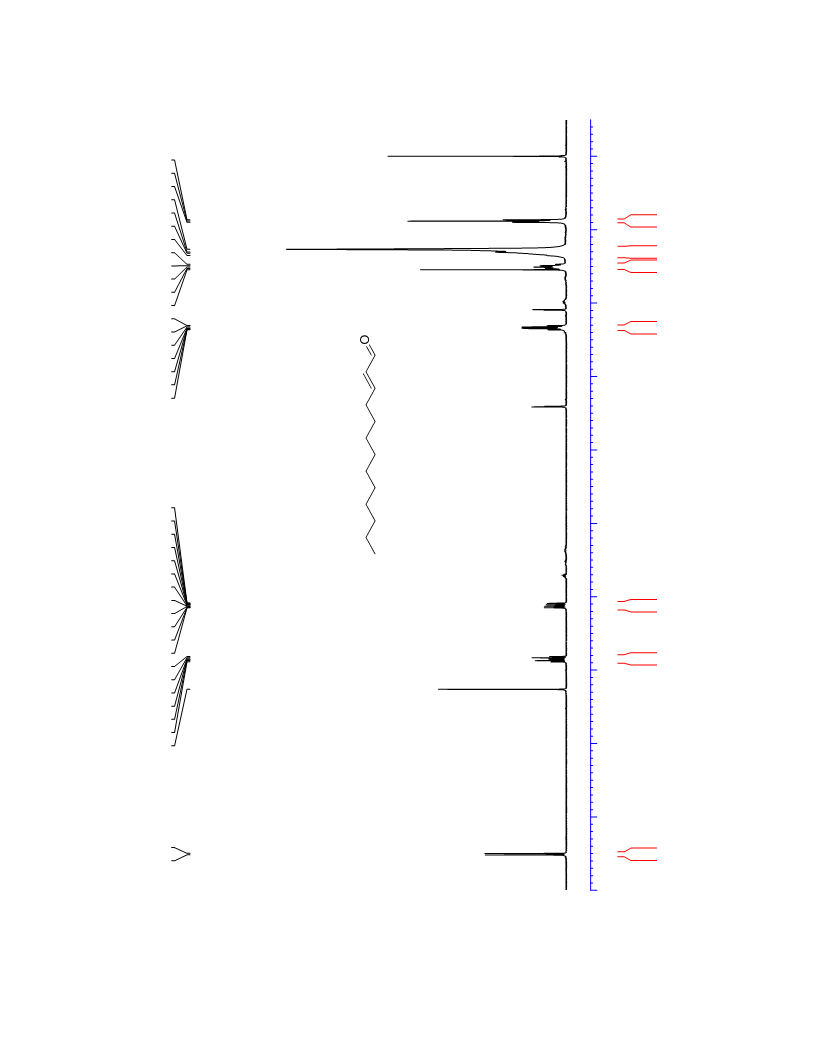
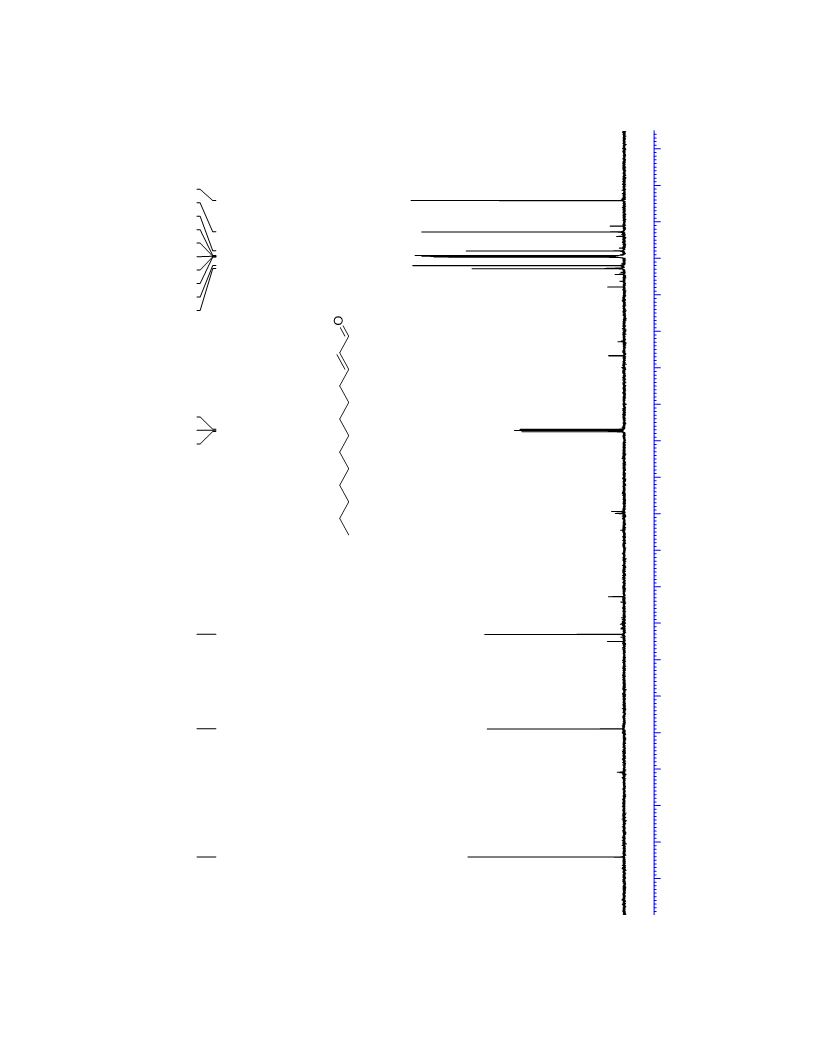
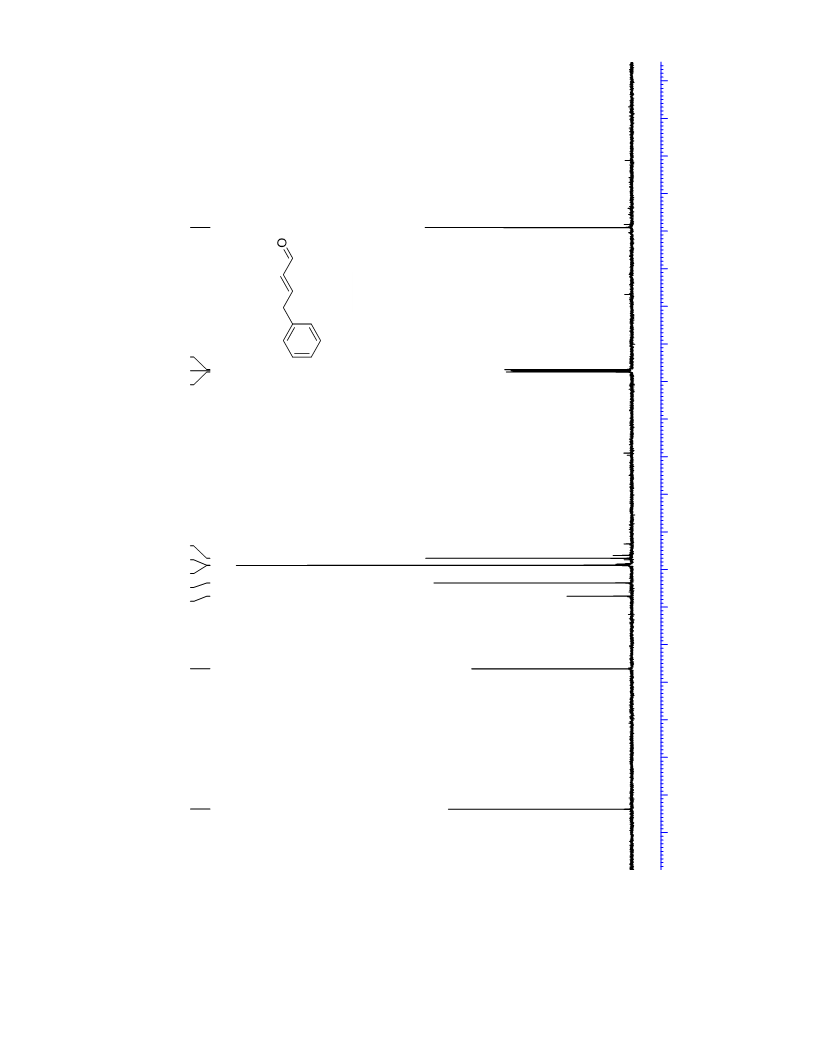
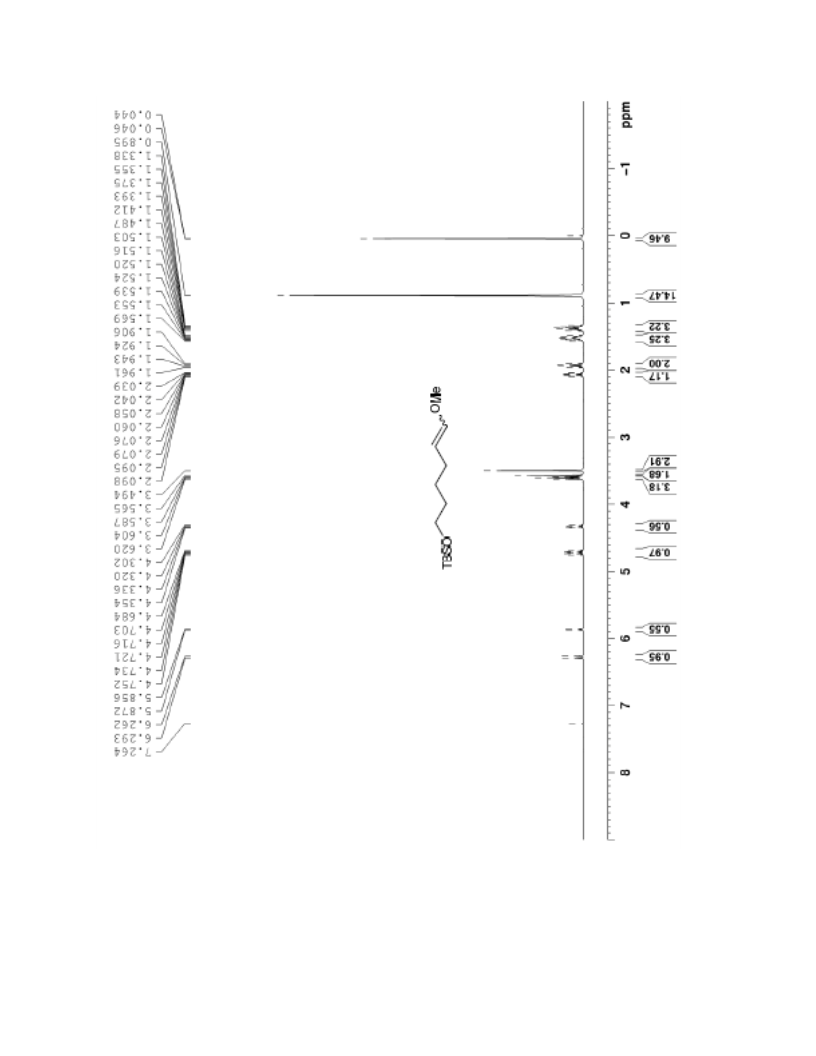
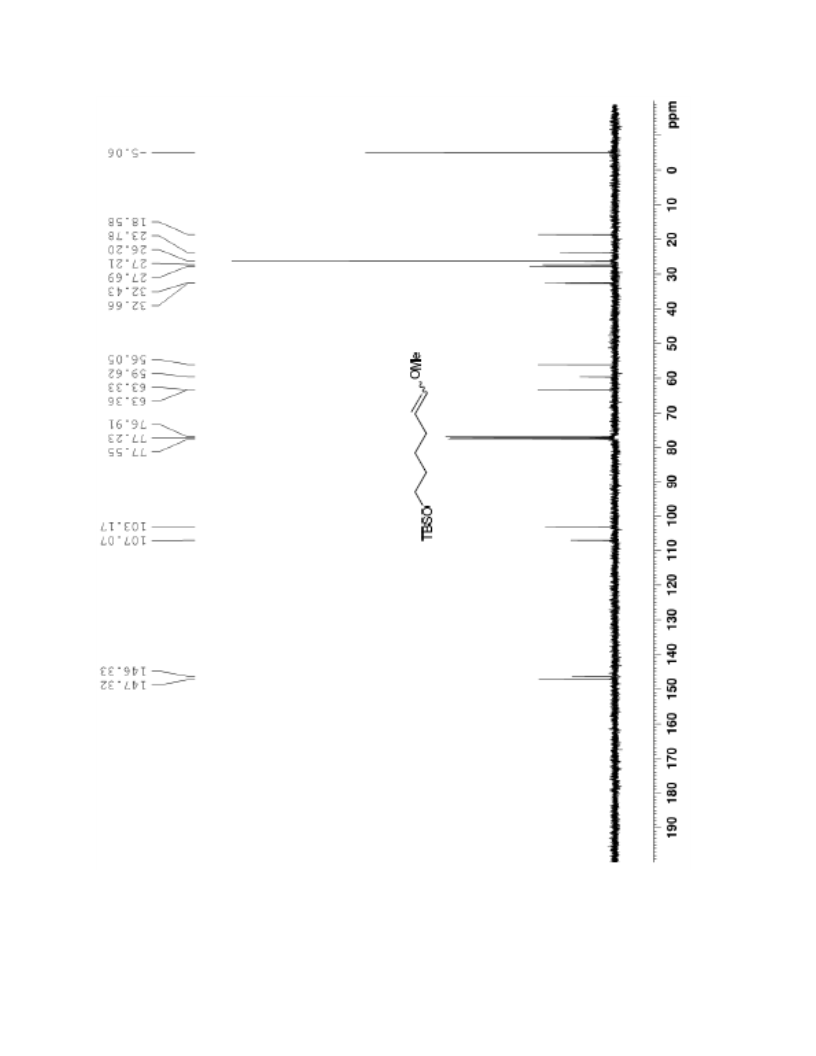
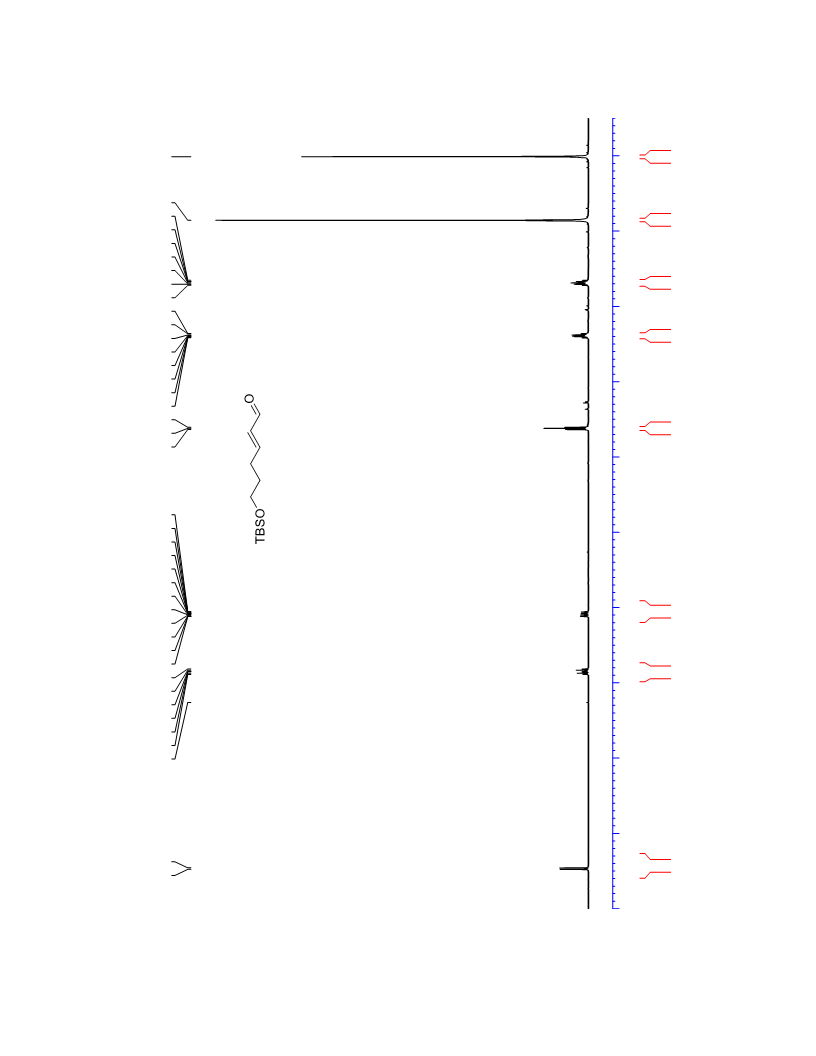
The palladium-catalyzed oxidation of alkyl enol ethers to enals, which employs low loadings of a palladium catalyst, is described. The mild oxidation conditions tolerate a diverse array of functional groups, while allowing the formation of di-, tri-, and tetrasubtituted olefins. The application of this methodology to intramolecular reactions of alkyl enol ethers containing pendant alcohols provides furan and 2,5-dihydrofuran products.
View More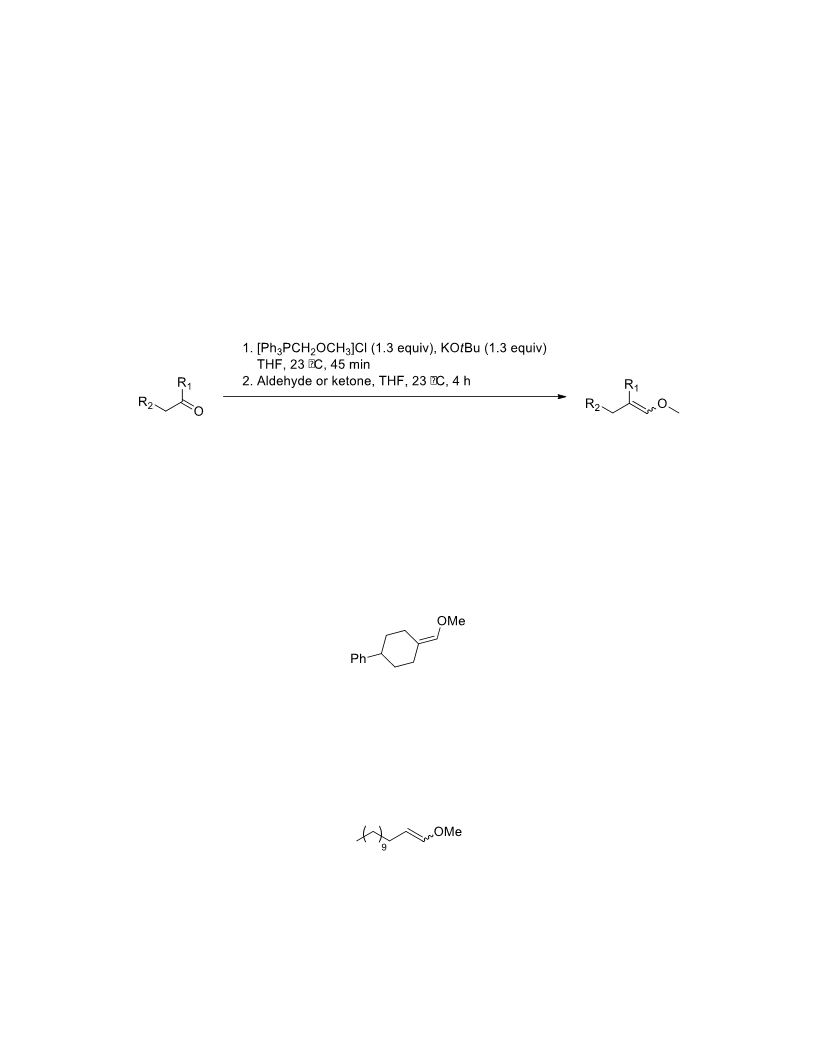













Shanghai Yurui Biotechnology(Anyang) Pharmaceutical Co., Ltd
Contact:+86-0372-3662335 +86-0372-3661988
Address:hanling industrial park anyang
Nanjing Chemzam Pharmtech Co., Ltd.
Contact:+86-25-86462165,+86-13915979898
Address:C5-1,6 Maiyue Road,Maigaoqiao,Nanjing,Jiangsu,China
Feiyang Biotechnology Co., Ltd.
Contact:+86-533-7866339
Address:Qilu Chemistry Park, 200m north of Management Community Building of Park
website:http://www.joyochem.com
Contact:0531-82687558, 0531-82687998
Address:Factory Building 11, Jinan Comprehensive free trade zone, Shandong, China
Changzhou Medi-tech Bioscientific Co., Ltd.(expird)
Contact:86-519-83246372
Address:Number 115 Menghedadao Road, Changzhou, Jiangsu, China
Doi:10.1055/s-0032-1316753
(2012)Doi:10.1016/j.molstruc.2021.131154
(2021)Doi:10.1016/j.tetlet.2012.09.096
(2012)Doi:10.1016/j.ejmech.2012.09.019
(2012)Doi:10.1248/cpb.42.1784
(1994)Doi:10.1016/j.bmc.2012.10.009
(2012)