
Bioorganic Chemistry (2019)
Update date:2022-08-11
Topics:
 Verma, Garima
Verma, Garima
 Khan, Mohemmed Faraz
Khan, Mohemmed Faraz
 Mohan Nainwal, Lalit
Mohan Nainwal, Lalit
 Ishaq, Mohd
Ishaq, Mohd
 Akhter, Mymoona
Akhter, Mymoona
 Bakht, Afroz
Bakht, Afroz
 Anwer, Tariq
Anwer, Tariq
 Afrin, Farhat
Afrin, Farhat
 Islamuddin, Mohammad
Islamuddin, Mohammad
 Husain, Ibraheem
Husain, Ibraheem
 Alam, Mohammad Mumtaz
Alam, Mohammad Mumtaz
 Shaquiquzzaman, Mohammad
Shaquiquzzaman, Mohammad
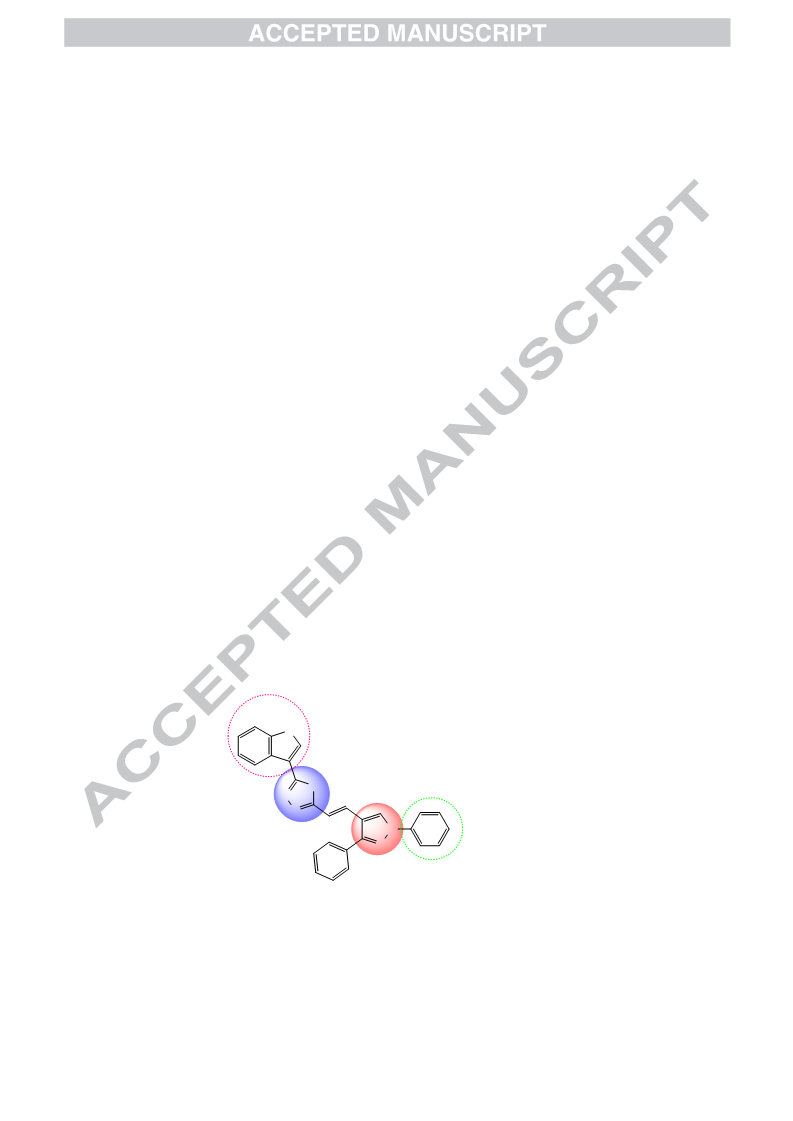
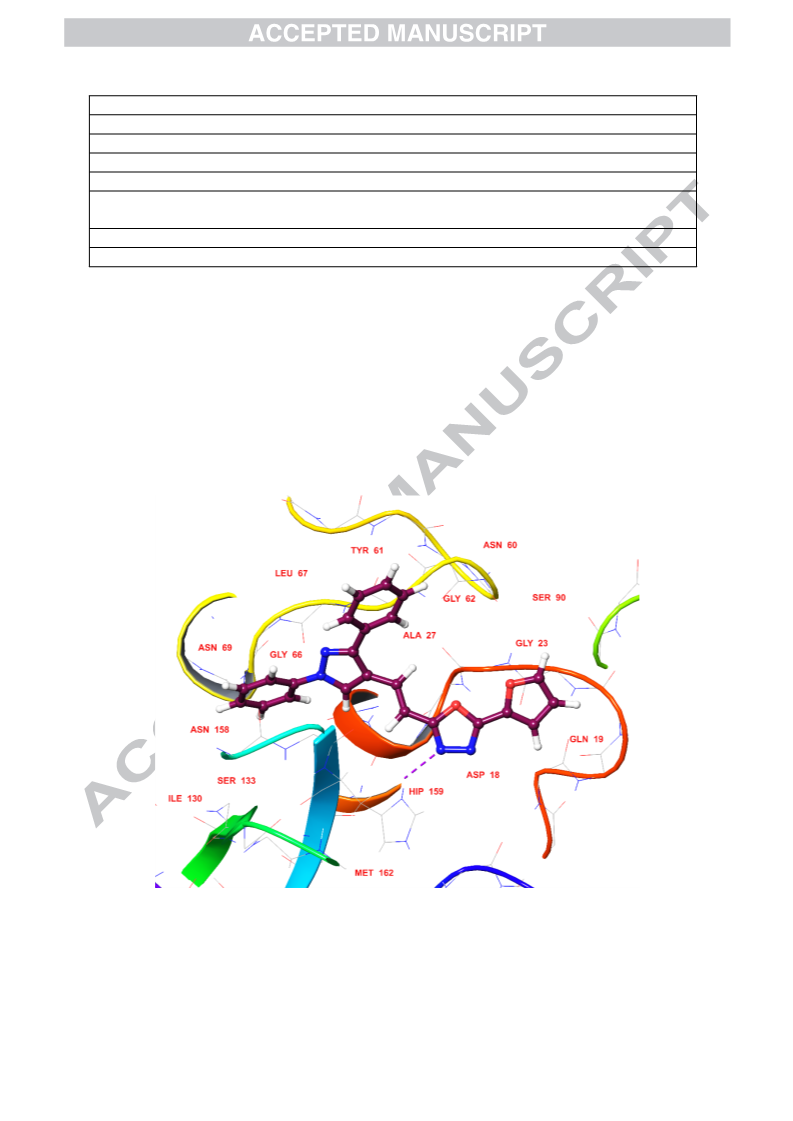
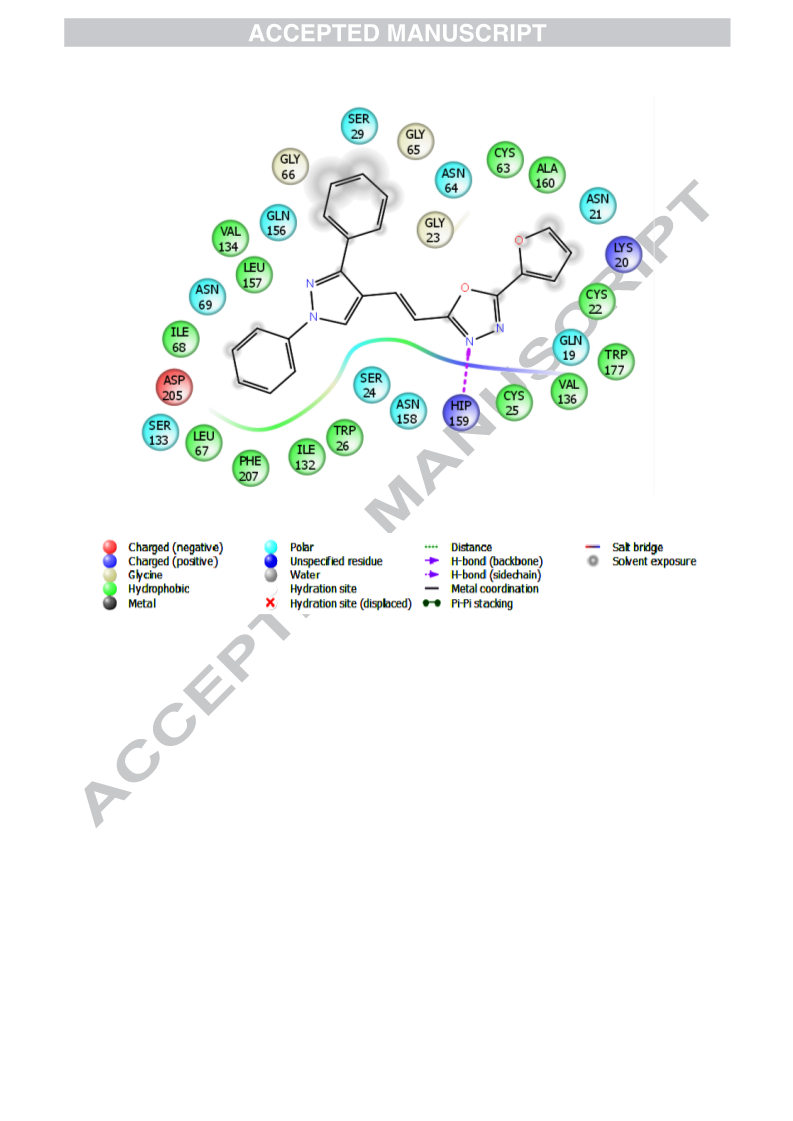
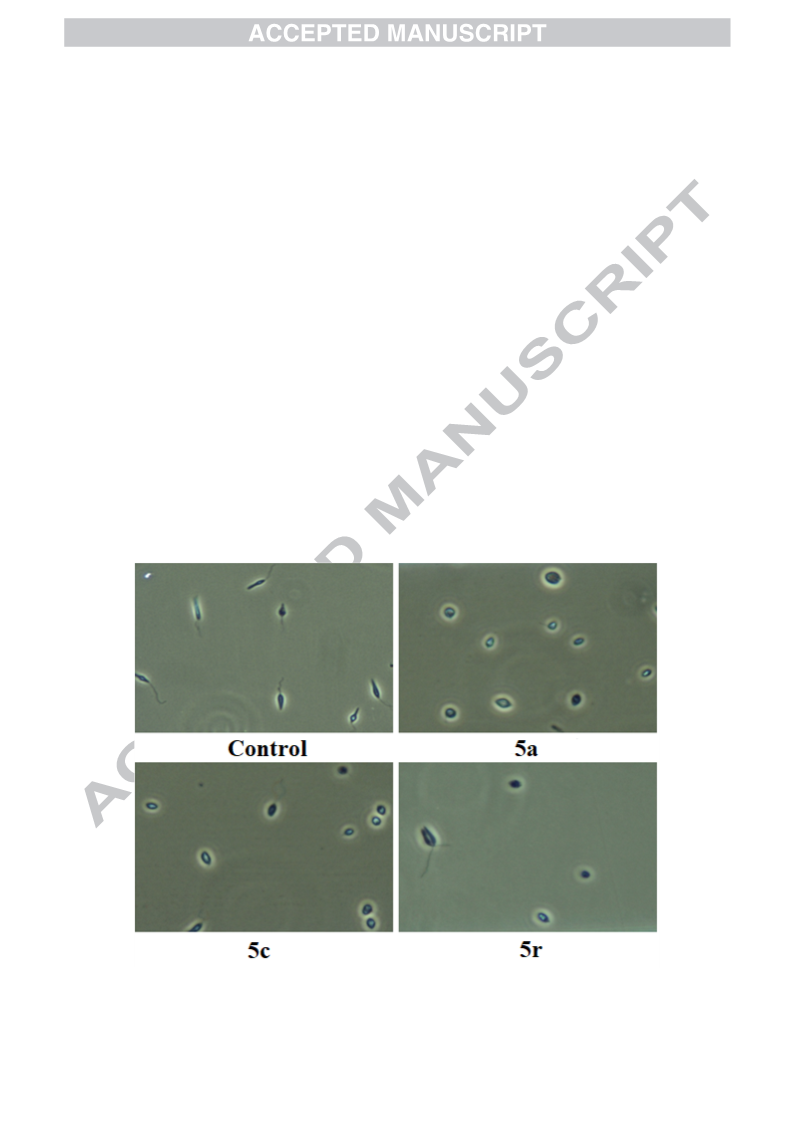
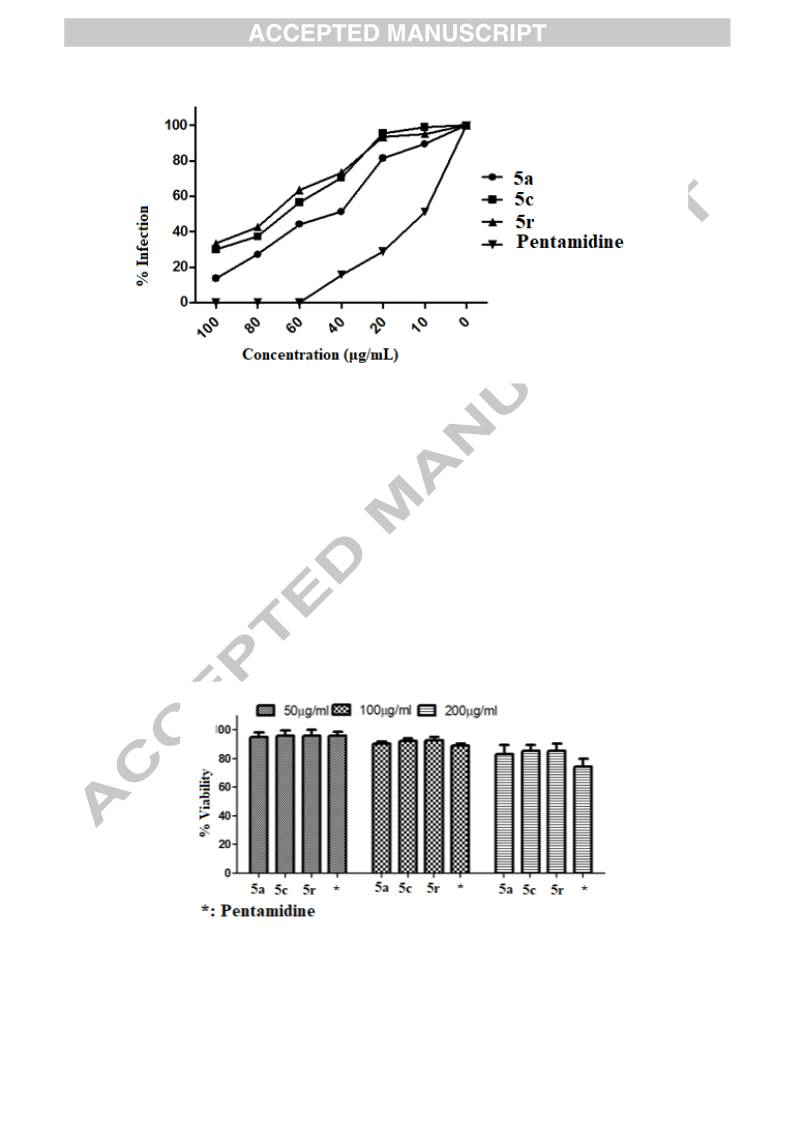
In continuance with earlier reported work, an extension has been carried out by the same research group. Mulling over the ongoing condition of resistance to existing antimalarial agents, we had reported synthesis and antimalarial activity of certain pyrazole-1,3,4-oxadiazole hybrid compounds. Bearing previous results in mind, our research group ideated to design and synthesize some more derivatives with varied substitutions of acetophenone and hydrazide. Following this, derivatives 5a–r were synthesized and tested for antimalarial efficacy by schizont maturation inhibition assay. Further, depending on the literature support and results of our previous series, certain potent compounds (5f, 5n and 5r) were subjected to Falcipain-2 inhibitory assay. Results obtained for these particular compounds further strengthened our hypothesis. Here, in this series, compound 5f having unsubstituted acetophenone part and a furan moiety linked to oxadiazole ring emerged as the most potent compound and results were found to be comparable to that of the most potent compound (indole bearing) of previous series. Additionally, depending on the available literature, compounds (5a–r) were tested for their antileishmanial potential. Compounds 5a, 5c and 5r demonstrated dose-dependent killing of the promastigotes. Their IC50 values were found to be 33.3 ± 1.68, 40.1 ± 1.0 and 19.0 ± 1.47 μg/mL respectively. These compounds (5a, 5c and 5r) also had effects on amastigote infectivity with IC50 of 44.2 ± 2.72, 66.8 ± 2.05 and 73.1 ± 1.69 μg/mL respectively. Further target validation was done using molecular docking studies. Acute oral toxicity studies for most active compounds were also performed.
View More























Contact:+86-710-3516804
Address:Number 83,Panggong road,Xiangcheng District,Xiangyang ,Hubei
Sichuan Sangao Biochemical Co., Ltd
Contact:+86-28-84874233
Address:19F, Bldg.2, Shudu Center, Tianfu 2nd St., Hi-tech zone, Chengdu 610041, Sichuan Province, China.
Evergreen Chemical Industry Ltd.
Contact:86-553-4918210
Address:6#2-602 Wanhaobailing
Jining Shengrun Chemical Industry Co., Ltd.
Contact:+86-537-7121666 ,
Address:West Ring Road,Wenshang County Shandong Province
Xian Changyue Biological Technology Co., Ltd.
website:https://www.xachangyue.com/
Contact:+86-029-62886900
Address:Keji Road NO.70
Doi:10.1016/S0040-4039(01)00548-2
(2001)Doi:10.1021/acs.orglett.7b02015
(2017)Doi:10.1039/c0dt00625d
(2010)Doi:10.1016/j.tet.2019.06.044
(2019)Doi:10.1021/jo00257a004
(1988)Doi:10.1039/c6ra23056c
(2016)