
Journal of Molecular Catalysis A: Chemical p. 76 - 82 (2016)
Update date:2022-08-17
Topics:
 Meng, Qingwei
Meng, Qingwei
 Zheng, Hongyan
Zheng, Hongyan
 Zhu, Yulei
Zhu, Yulei
 Li, Yongwang
Li, Yongwang
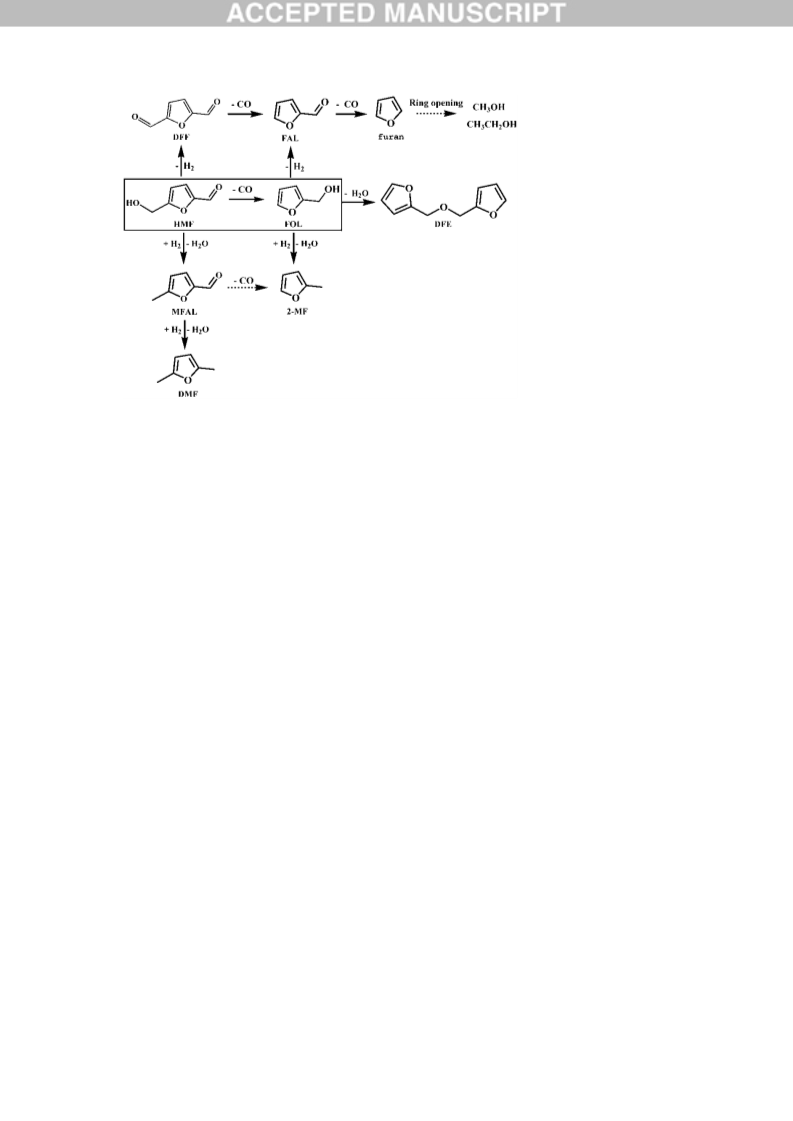
An extensive product distribution is firstly examined in the process of 5-hydroxymethylfurfural (HMF) decarbonylation over Pd-based catalysts and some interesting results are obtained. The main side reactions are due to the high activity of the furan ring-branched hydroxymethyl, which could go through hydrogenolysis, dehydrogenation and etherification. The H2 source was carefully explored and determined to be the hydroxymethyl dehydrogenation. The reactivity of the main intermediates was separately investigated and their evolution pathway was obtained. Noticeably, it is demonstrated that the elimination of the furanic ring-branched hydroxymethyl (in HMF or furfuryl alcohol) is completed by sequential dehydrogenation and decarbonylation via the intermediate of aldehyde (2, 5-diformylfuran or furfural). A comprehensive reaction pathway for HMF decarbonylation is proposed, which is significant for designing highly selective decarbonylation catalysts.
View More























shanghai tuomiao chemicial co.,ltd.
website:https://www.tuomiaochem.com/
Contact:021 - 59853336
Address:shanghai
Shenyang Xingzhenghe Chemical Co., Ltd.
Contact:024-23509232
Address:No. 33, Naner Road, Heping Dist.
Xinchang Yueding Chemical Co., Ltd.
Contact:86-571-56926323
Address:NO.90 BEIMENCHENGWAI CHENGGUAN TOWN XINCHANG
zhuzhou zhongle chemical co. ltd.
Contact:+86-0731 28228409
Address:Zhuzhou, Hunan, China
FOSHAN NANHAI ZHONGNAN PHARMACEUTICAL FACTORY
Contact:0086-0757-85609331
Address:XIAHENGTIAN INDUSTRIAL ZONE,SHAYONG VILLAGE,LISHUI TOWN
Doi:10.1021/jo01269a111
(1968)Doi:10.1039/C19680000440
(1968)Doi:10.1177/1747519819901251
(2020)Doi:10.1246/cl.1981.1169
(1981)Doi:10.1016/j.apcata.2013.05.022
(2013)Doi:10.1016/j.phytochem.2012.08.019
(2013)