
Journal of Physical Organic Chemistry p. 585 - 592 (2009)
Update date:2022-08-10
Topics:
 Jarmoumi, Chakir
Jarmoumi, Chakir
 Lakhrissi, Brahim
Lakhrissi, Brahim
 Mondieig, Denise
Mondieig, Denise
 Negrier, Philippe
Negrier, Philippe
 Leger
Leger
 Massip
Massip
 Lazard, Zhor
Lazard, Zhor
 Benali, Bouziane
Benali, Bouziane
 Massoui, Mohamed
Massoui, Mohamed
 Essassi, El Mokhtar
Essassi, El Mokhtar
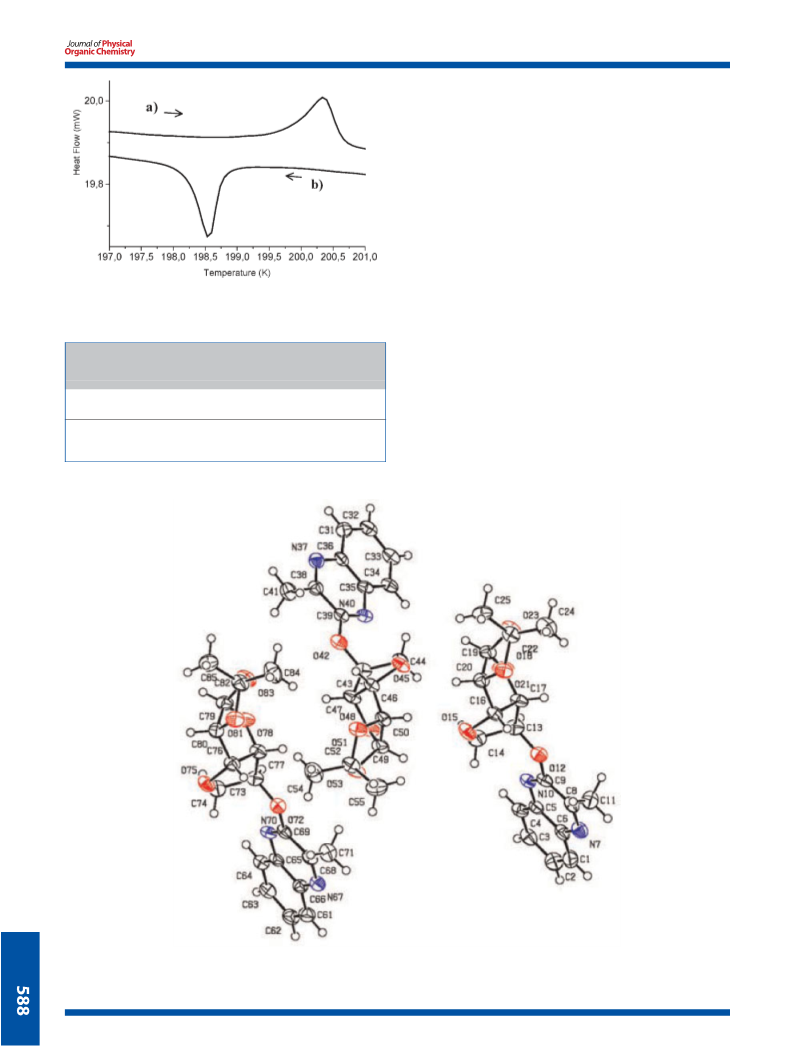
Reaction of 3-methyl-2(1H)-quinoxalinone (4) and 2(1H)-quinoxalinone (5) with 5,6-anhydro-1,2-O-isopropylidene-α-D-glucofuranose 6 gives the unexpected O-glucoquinoxalines derivatives by the intermediary novel intramolecular rearrangement of 5,6-anhydro-1,2-O-isopropylidene-α-D- glucofuranose to the corresponding 3,6-anhydro form. The obtained O-glucoquinoxalines 7,8 were identified by NMR spectroscopy. The X-ray crystal structures have been determined at room temperature. Moreover, a solid-solid phase transition has been detected at 198.9 K for O-glucoquinoxalines 7 and the structure of the low-temperature phase has been solved at 188K.
View More






Contact:886 2 2541 0022
Address:8 Fl., No. 11, Sec. 1, Chung Shan North Rd., Taipei, Taiwan R.O.C.
Weifang Adde Economic And Trade Co.,LTD.
Contact:86-536-8885548
Address:Room 1402,Wanda Plaza B Block,No.958,Yuanfei Road,Kuiwen District
Laohekou Jinghong Chemical Co.,Ltd
Contact:+86-0710-3702747
Address:163.East,Huagong Road,Laohekou
Shandong Xiangde Biotechnology Co., Ltd
Contact:+86 -15066639877
Address:Sanba street
NINGBO PANGS CHEM INT’L CO.,LTD.
Contact:+86-574-27666845
Address:FLOOR 21,BUILDING NO.11,XIN TIAN DI,NO.689 SHI JI ROAD,NINGBO CHINA
Doi:10.1016/j.poly.2014.06.002
(2014)Doi:10.1016/S0040-4039(00)00165-9
(2000)Doi:10.1016/j.chemphyslip.2016.08.004
(2016)Doi:10.1002/1521-3773(20020301)41:5<777::AID-ANIE777>3.0.CO;2-7
(2002)Doi:10.1021/ja01107a038
(1953)Doi:10.1016/0957-4166(94)80074-X
(1994)