
ACS Catalysis p. 5812 - 5821 (2015)
Update date:2022-08-16
Topics:
 Vidal, Juan D.
Vidal, Juan D.
 Climent, Maria J.
Climent, Maria J.
 Concepcion, Patricia
Concepcion, Patricia
 Corma, Avelino
Corma, Avelino
 Iborra, Sara
Iborra, Sara
 Sabater, Maria J.
Sabater, Maria J.
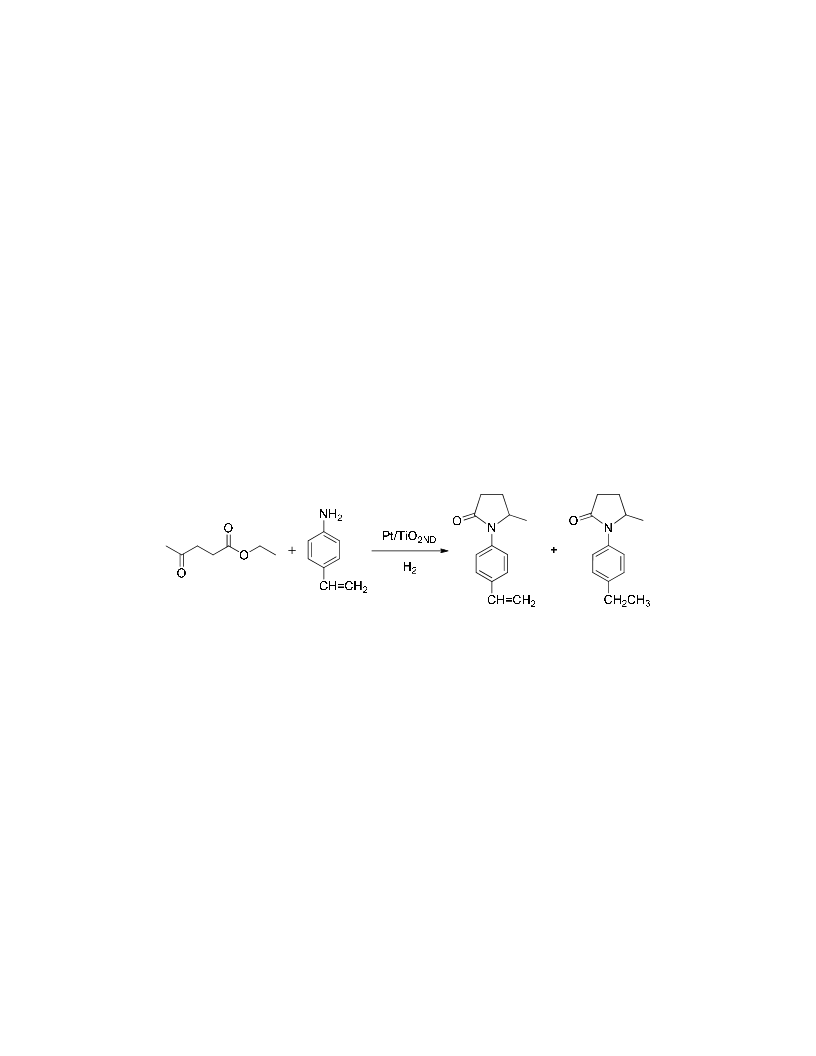
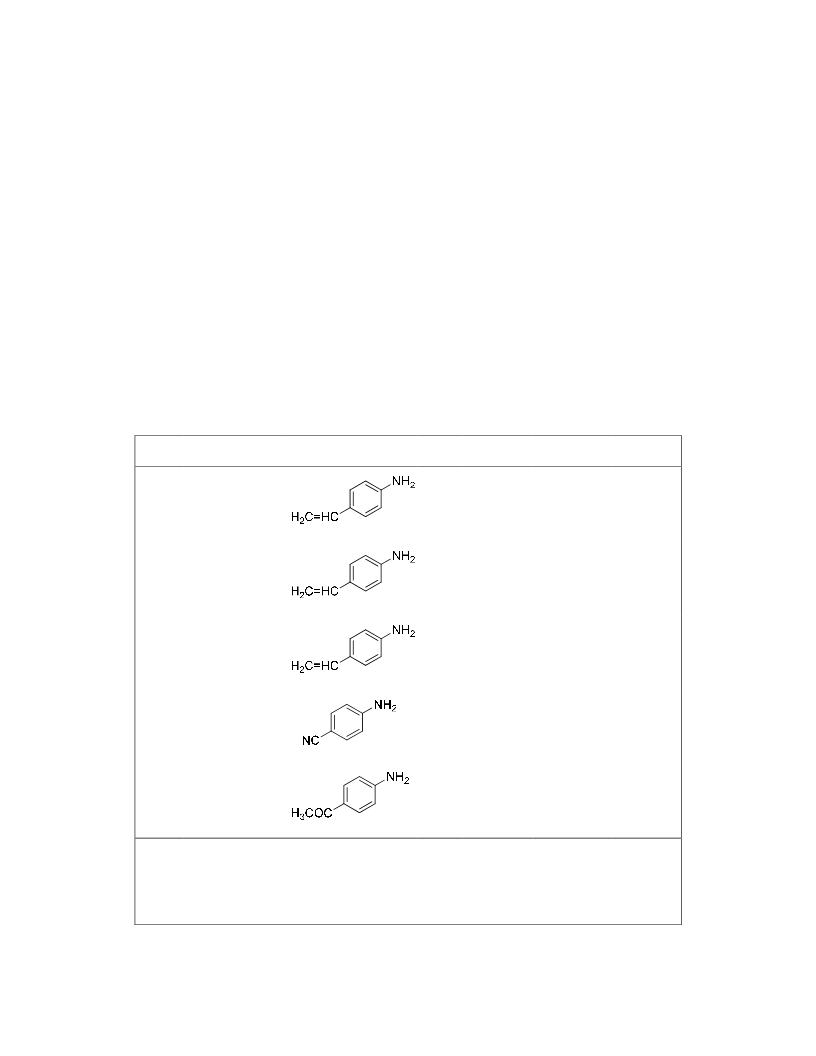
N-substituted-5-methyl-2-piyrrolidones have been obtained by reductive amination of ethyl levulinate with amines in the presence of H2 as reducing agent under solvent-free conditions. The process involves as a first step the formation of an imine intermediate followed by hydrogenation of the imine group and subsequent cyclization into pyrrolidone. Pt/TiO2 with Pt crystal faces decorated with TiOx is a very active and chemoselective catalyst, being possible to achieve high conversion and selectivity to the corresponding N-substituted-5-methyl-2-pyrrolidones even when other groups susceptible of hydrogenation such as vinyl, carbonyl, or cyano groups are present in the amine moiety. A kinetic study showed that the reaction-controlling step is the formation of the imine intermediate. The rate of formation is enhanced by the presence of protonic acid sites generated on the support by hydrogen dissociation on the metal, resulting in a true bifunctional catalyst for the reaction.
View More





Dayang Chem (Hangzhou) Co.,Ltd.
website:http://www.dycnchem.com
Contact:+86-571-88938639
Address:9/F, Unit 2 Changdi Torch Building, 259# WenSan Road, Xihu District, Hangzhou City 310012, P.R.China
Contact:86-371-63655023
Address:No.85,jinshui road,zhengzhou,China
Wuhan Benjamin Pharmaceutical Chemical Co.,Ltd
Contact:86-27-52341789
Address:Room 1518 B suite, optical valley time square, No 111 Guanshan Road, Hongshan District,Wuhan,Hubei Province,China.
Anhui Redstar Pharmaceutical Corp., Ltd
Contact:+86-563-5120837
Address:Jingxian Industrial Development Zone, Anhui , China
Nanjing Xi Ze Biological Technology Co., Ltd
Contact:86-025-66023220
Address:Address: Nanjin Qixia District Maigaoqiao R & D base in Pioneer Park Chun Yin Road 18-A928
Doi:10.1039/c3ra43022g
(2013)Doi:10.1016/j.tetlet.2019.03.038
(2019)Doi:10.1055/s-2005-861877
(2005)Doi:10.1002/cssc.201402466
(2014)Doi:10.1039/d1cc00093d
(2021)Doi:10.1021/acs.inorgchem.5b00435
(2015)