
Journal of the American Chemical Society p. 6466 - 6472 (1980)
Update date:2022-08-12
Topics:
 Palmer, John L.
Palmer, John L.
 Jencks, William P.
Jencks, William P.
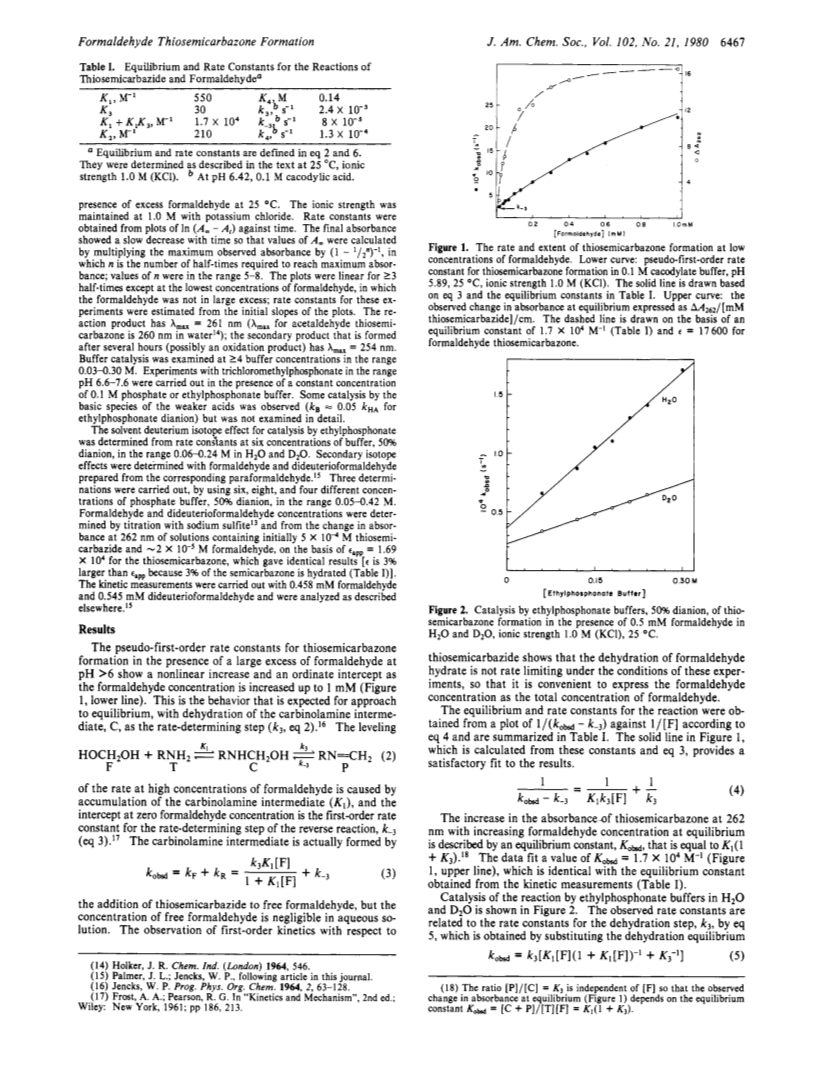
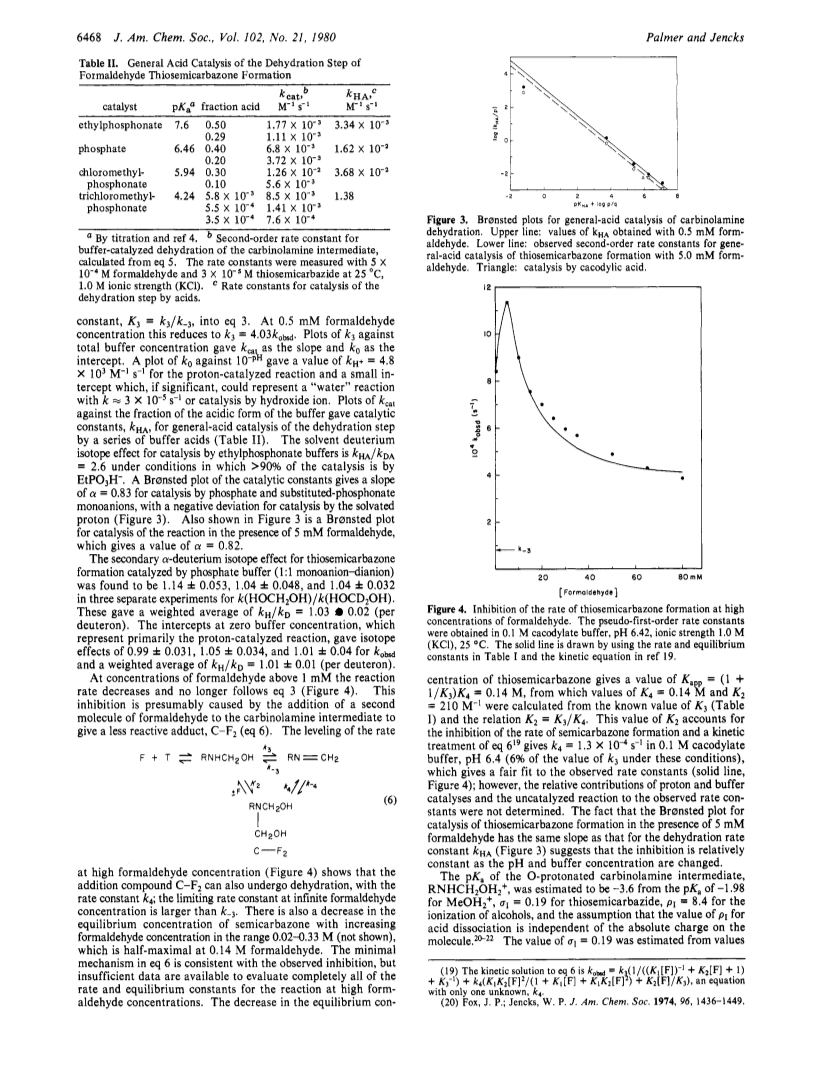
At pH>6 the formation of formaldehyde thiosemicarbazone proceeds with rate-limiting dehydration of the carbinolamine intermediate, which is at equilibrium with formaldehyde hydrate and thiosemicarbazide (K = 550 M-1).At higher concentrations of formaldehyde a bis(formaldehyde) addition compound is formed, which undergoes dehydration more slowly.The dehydration step is subject to general-acid catalysis by phosphate and phosphonate buffers with α = 0.83.A solvent deuterium isotope effect of kHA/kDA = 2.6 for catalysis by ethylphosphonate monoanion and published evidence support a concerted mechanism of catalysis.The calculated rate constant for formation of the O-protonated carbinolamine is > 104 faster than the observed rate constant for dehydration and the rate constant for expulsion of water from this species is < 3 x 107 s-1.Thus, it appears that a concerted mechanism can exist when it is not enforced by the nonexistence of the O-protonated species.The secondary α-deuterium isotope effect of KH/kD = 1.06 (1.03 /D) for catalysis by phosphate monoanion suggests an early transition state but other criteria suggest a central or late transition state for C-O cleavage.
View More

Hangzhou GreenCo Science & Technology Co., Ltd.
Contact:86-571-88257303
Address:1713 Room,Jingui Building,Gudun Road,Xihu District,Hangzhou,China
Shandong Hongxiang Zinc Co., Ltd
Contact:086-0311-66187879
Address:DaWang developing zone
Jinan Baozhao Pharmaceutical Co., Ltd
Contact:0086-531-86397156 82371858 82371868
Address:Huaneng Road, Jinan, Shandong ,China
Yixing Bluwat Chemicals Co., Ltd.
Contact:+86 510 87821568
Address:Yongan Road, Yixing Chemical Industrial Park, Yixing, Jiangsu, China
KINHENG CHEMICAL(SHANGHAI)CO., LTD.
Contact:+8621-60490170
Address:Room401, No.28,Lane 189, Yangshupu Rd. Shanghai, China.
Doi:10.1016/j.molcata.2005.09.011
(2006)Doi:10.1029/1999GL011251
(1936)Doi:10.1016/j.jcat.2012.08.008
(2012)Doi:10.1016/j.tetlet.2010.10.134
(2011)Doi:10.1016/S0022-328X(00)80606-3
(1976)Doi:10.1016/j.electacta.2015.01.074
(2015)