
Heterocycles p. 169 - 194 (1999)
Update date:2022-08-11
Topics:
 Honty, Katalin
Honty, Katalin
 Demeter, ádám
Demeter, ádám
 Szántay Jr., Csaba
Szántay Jr., Csaba
 Hollósi, Miklós
Hollósi, Miklós
 Kolonits, Pál
Kolonits, Pál
 Szántay, Csaba
Szántay, Csaba
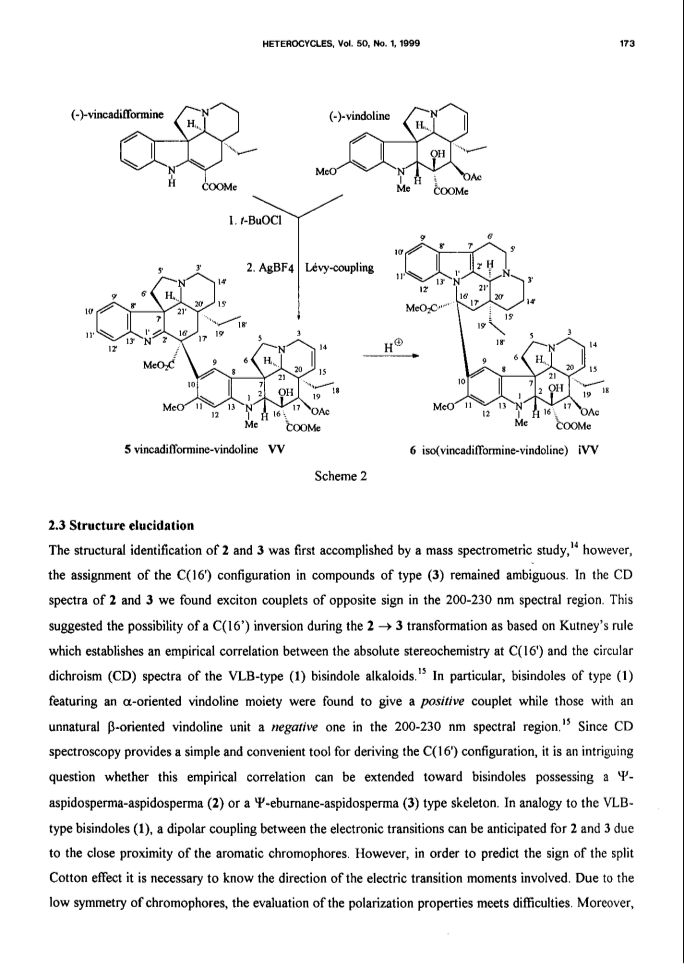
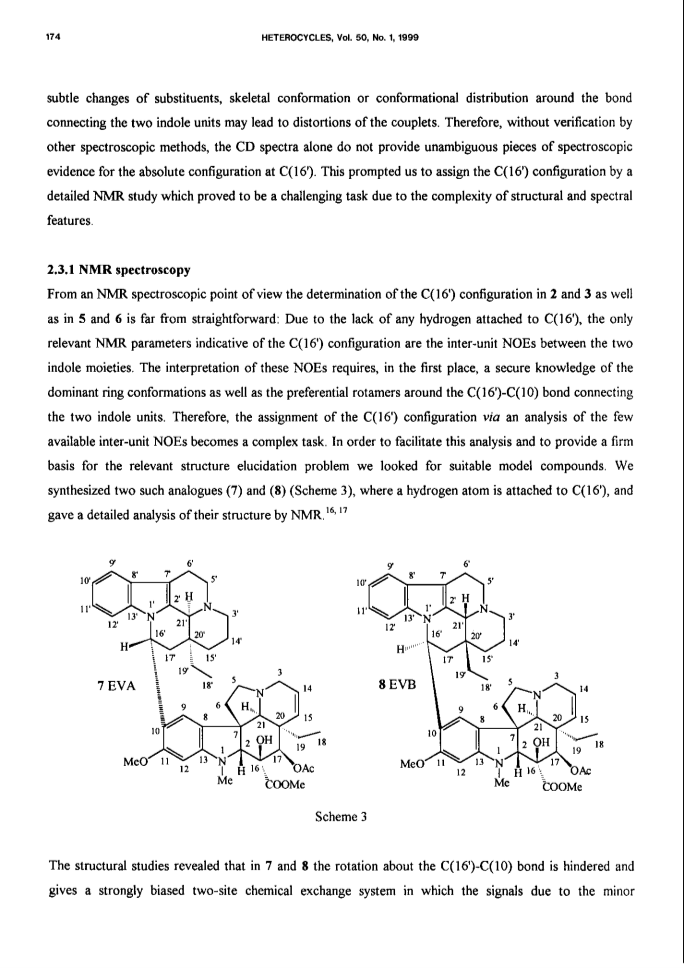
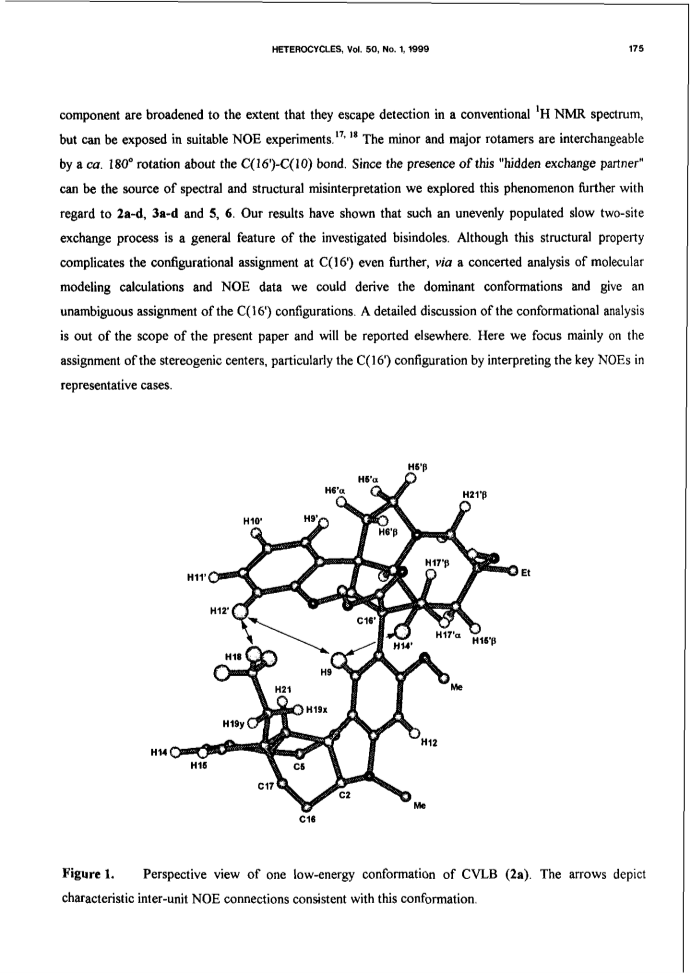
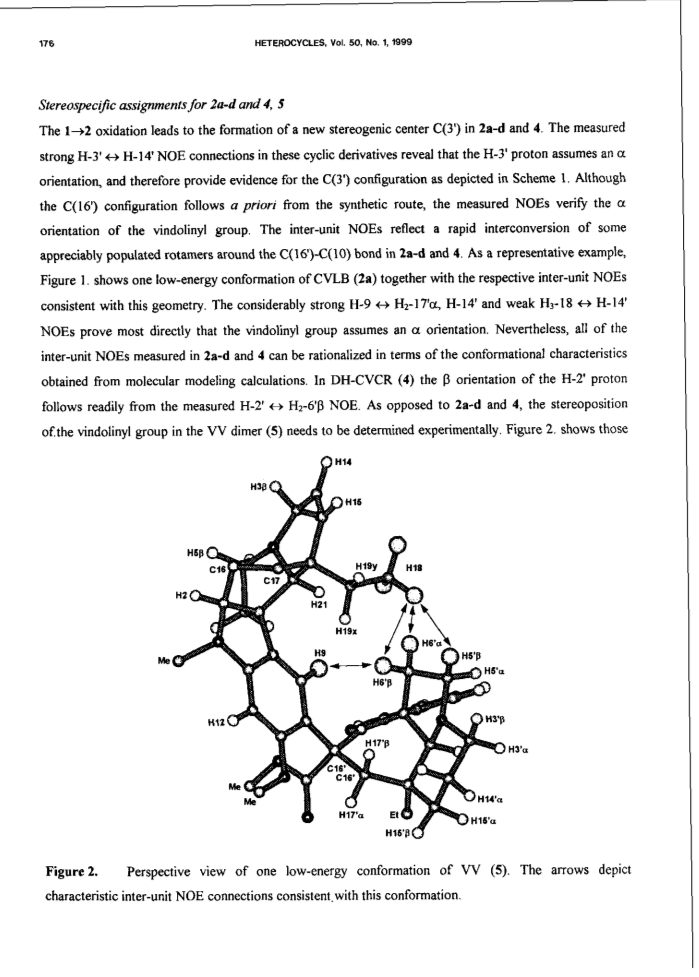
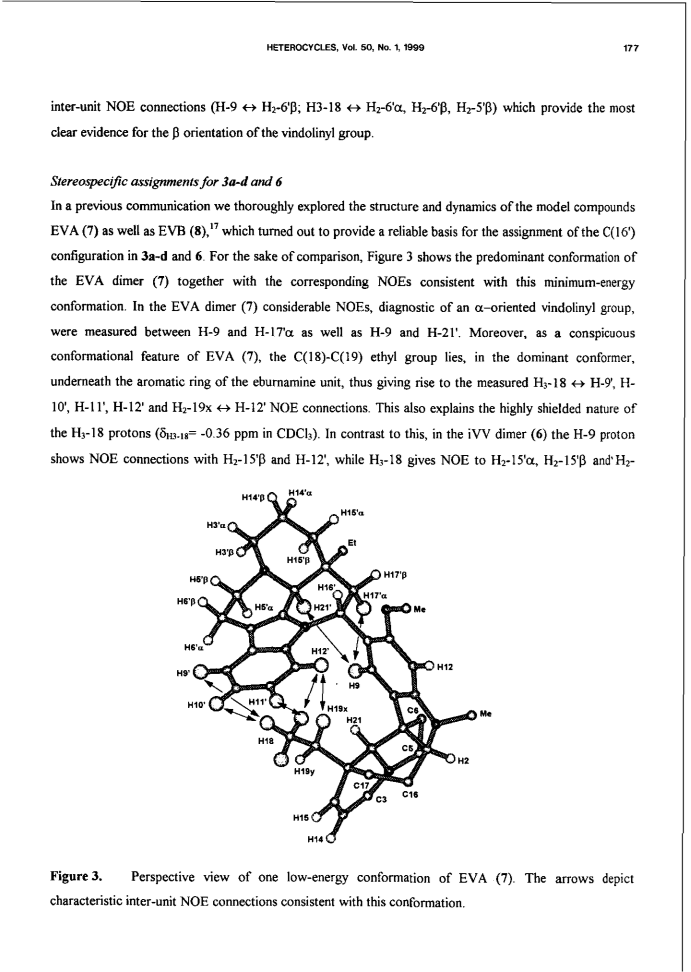
Bisindole alkaloids of the vinblastine (VLB) type can be oxidized to give a Ψ-aspidosperma-aspidosperma type skeleton via 3'-7'-transannular cyclization. Acid catalysis triggers an aspidospermane→eburnane skeletal rearrangement of these cyclic derivatives, thus giving a novel bisindole system with a Ψ-eburnea-aspidosperma type skeleton. A previously unexplored aspect of this transformation is the observed retention or inversion at C(16') depending on the starting C(16') configuration. The present paper gives a detailed account of the synthetic aspect of this work together with preliminary NMR and CD results concerning the epimerization at C(16').
View More




















Chongqing Rong&Quan Pharmaceutical Technology Co. , Ltd.
Contact:86-023-65268721
Address:No. 7, Manshanhong Village, Pingdingshan, Shapingba District, Chongqing Province, China
Jiangxi Lanqi Fine Chemical S&T Co., Ltd.
Contact:+86-21-64891143
Address:XinJiShan Industrial Area, Zhangshu City, JiangXi Province, China
Jiangxi Huashi Pharmaceutical Co., Ltd
Contact:+86-795-4509628
Address:Ningbo Ave., Fengtian Industrial Park, Fengxin Country, Jiangxi, China.
Beijing Apis Biotechnology Co., Ltd.
Contact:86-010-67856775-8551
Address:NO.4PUHUANGYU ROAD,FENTAI DISTRICT, BEIJING, CHINA
website:http://www.uvchemkeys.com
Contact:0086-021-58785816
Address:RM2607 Building No.1 Guosheng, Lane 388, Zhongjiang Road, Putuo District, Shanghai 200062 China
Doi:10.1016/S0022-1139(00)85128-3
(1983)Doi:10.1039/jr9300002267
(1930)Doi:10.1016/j.bioorg.2018.06.010
(2018)Doi:10.1007/s10870-011-0001-2
(2011)Doi:10.1016/j.saa.2021.119438
(2021)Doi:10.1021/ja00353a069
(1983)