Synthesis of metabolites of dapagliflozin: an SGLT2 inhibitor
-
Source and publish data:
Journal of Chemical Sciences (2020)
Update date:2022-08-30
Topics:
-
Authors:
 Bonige, Kishore Babu
Bonige, Kishore Babu
 Chavakula, Ramadas
Chavakula, Ramadas
 Karumanchi, Kishore
Karumanchi, Kishore
 Korupolu, Raghu Babu
Korupolu, Raghu Babu
 Natarajan, Senthil Kumar
Natarajan, Senthil Kumar
 Peruri, Badarinadh Gupta
Peruri, Badarinadh Gupta
Article abstract of DOI:10.1007/s12039-020-1747-x
Abstract: Dapagliflozin is one of the gliflozin class drugs, useful for the treatment of type-2 diabetes. Dapagliflozin undergoes extensive metabolism and transforms to metabolites in humans. The contribution of pharmacologically active metabolites in drug discovery and development is significant. A streamlined synthetic approach is devised to access three metabolites of dapagliflozin namely, benzylic hydroxy dapagliflozin, oxo dapagliflozin and desethyl dapagliflozin. Two synthetic protocols have been proposed for the synthesis of benzylic hydroxy dapagliflozin and oxo dapagliflozin. An enantioselective deethylation of dapagliflozin is also reported. Graphic abstract: Synthesis of three metabolites of Dapagliflozin namely benzylic hydroxy dapagliflozin, oxo dapagliflozin and desethyl dapagliflozin is reported from commercially accessible raw materials.[Figure not available: see fulltext.]
View More
Full text of DOI:10.1007/s12039-020-1747-x

J. Chem. Sci.
(
2
0
2
0
)
1
3
2
:
4
2
Ó
I
n
d
i
a
n
A
c
a
d
e
m
y
o
f
S
c
i
e
n
c
e
s
S
a
d
h
a
n
(
0
1
2
3
4
5
6
7
8
9
(
)
.
v
o
l
V
)
F
T
3
]
(
0
1
2
3
4
5
6
(
)
.
v
o
l
V
)
RAPID COMMUNICATION
Synthesis of metabolites of dapagliflozin: an SGLT2 inhibitor
a
,
b
,
a
*
K
I
S
M
D
H
A
A
O
D
R
R
E
A
N
K
A
H
H
R
A
U
V
G
M
A
U
A
K
N
U
A
C
L
H
I
,
S
H
E
U
N
B
T
A
H
B
I
L
U
K
K
U
O
M
R
A
U
R
N
L
A
U
T
,
A
K
R
I
A
S
J
H
A
O
N
,
a
A
b
b
R
A
S
C
,
R
U
A
R
G
P
O
R
E
B
A
B
U
B
O
N
I
G
E
a
n
d
c
B
A
I
A
D
P
T
P
E
R
I
a
C
h
e
m
i
c
a
l
R
e
s
e
a
r
c
h
a
n
d
D
e
v
e
l
o
p
m
e
n
t
,
A
P
L
R
e
s
e
a
r
c
h
C
e
n
t
r
e
-
I
I
,
A
u
r
o
b
i
n
d
o
P
h
a
r
m
a
L
t
d
.
,
S
a
u
r
v
e
y
N
o
.
7
1
&
7
b
2
,
I
n
d
r
a
k
a
r
a
n
V
i
l
l
a
g
e
,
K
a
n
d
i
M
a
n
d
a
l
,
S
,
a
n
g
a
r
e
d
d
y
D
i
s
t
r
i
c
t
,
T
e
l
a
n
g
a
n
a
S
t
a
t
e
5
0
2
3
2
a
9
,
I
n
d
i
D
e
p
a
r
t
m
e
n
t
o
f
E
n
g
i
n
e
e
r
i
n
g
C
h
e
m
i
s
t
r
y
A
.
U
.
C
o
l
l
e
g
e
o
f
E
n
g
i
n
e
e
r
i
n
g
(
A
)
,
A
n
d
h
r
U
n
i
v
e
r
s
i
t
y
,
V
i
s
a
k
h
a
p
a
t
n
a
m
,
A
n
d
h
r
a
P
r
a
d
e
s
h
5
3
0
0
0
3
,
I
n
d
i
a
c
A
n
a
l
y
t
i
c
a
l
R
e
s
e
a
r
c
h
a
n
d
D
e
v
e
l
o
p
m
e
n
t
,
A
P
L
R
e
s
e
a
r
c
h
C
e
n
t
r
e
-
I
I
,
A
u
r
o
b
i
n
d
o
P
h
a
r
m
a
L
t
d
,
S
a
u
r
v
e
y
N
o
.
7
1
&
7
2
,
I
n
d
r
a
k
a
r
a
n
V
i
l
l
a
g
e
,
K
m
a
n
d
i
M
a
n
d
a
l
,
S
a
n
g
a
r
e
d
d
y
D
i
s
t
r
i
c
t
,
T
e
l
a
n
g
a
n
a
S
t
a
t
e
5
0
2
3
2
9
,
I
n
d
i
E
-
m
a
i
l
:
k
k
i
s
h
o
r
e
0
0
9
@
g
a
i
l
.
c
o
m
M
S
r
e
c
e
i
v
e
d
7
J
u
l
y
2
0
1
9
;
r
e
v
i
s
e
d
2
1
D
e
c
e
m
b
e
r
2
0
1
9
;
a
c
c
e
p
t
e
d
5
J
a
n
u
a
r
y
2
0
2
0
Abstract.
g
D
e
a
p
a
g
l
i
fl
o
z
i
n
i
s
o
n
e
o
f
t
h
e
g
l
i
fl
o
zi n c la ss d ru gs , u se fu l f or t he t re at me nt o f t yp e- 2 d ia be te s. D ap a-
h
l
i
fl
o
z
i
n
u
n
d
r
g
o
e
s
e
x
t
e
n
s
i
v
e
m
e
t
a
b
o
l
i
s
m
a
n
d
t
r
a
n
s
f
o
r
m
s
t
o
m
e
t
a
b
o
l
i
t
e
s
i
n
u
m
a
n
s
.
T
h
e
c
o
n
t
r
i
b
u
t
i
o
n
o
f
p
h
a
r
-
m
a
c
o
l
o
g
i
c
a
l
l
y
a
c
t
i
v
e
m
e
t
a
b
o
l
i
t
e
s
i
n
d
r
u
g
d
i
s
c
o
v
e
r
y
a
n
d
d
e
v
e
l
o
p
m
e
n
t
i
s
s
i
g
n
i
fi
c
a
n
t
.
A
s
t
r
e
a
m
l
i
n
e
d
s
y
n
t
h
e
o
y
t
i
c
a
d
h
p
p
r
o
a
c
h
i
s
d
e
v
i
s
e
d
t
o
a
c
c
e
s
s
t
h
r
e
e
m
e
t
a
b
o
l
i
t
e
s
o
f
d
a
p
a
g
l
i
fl
o
z
i
n
n
a
m
e
l
y
,
b
e
n
z
y
l
i
c
h
y
d
r
o
x
y
d
a
p
a
g
l
i
fl
o
z
i
n
,
x
o
a
p
a
g
l
i
fl
o
z
i
n
a
n
d
d
e
s
e
t
h
y
l
d
a
p
a
g
l
i
fl
o
z
i
n
.
T
w
o
s
y
n
t
h
e
t
i
c
p
r
o
t
o
c
o
l
s
h
a
v
e
b
e
e
n
p
r
o
p
o
s
e
d
f
o
r
t
h
e
s
y
n
t
h
e
s
i
s
o
f
b
e
n
z
l
i
c
y
d
r
o
x
y
d
a
p
a
g
l
i
fl
o
z
i
n
a
n
d
o
x
o
d
a
p
a
g
l
i
fl
o
z
i
n
.
A
n
e
n
a
n
t
i
o
s
e
l
e
c
t
i
v
e
d
e
e
t
h
y
l
a
t
i
o
n
o
f
d
a
p
a
g
l
i
fl
o
z
i
n
i
s
a
l
s
o
r
e
p
o
r
t
e
d
.
Keywords.
Abbreviations
D
a
p
a
g
l
i
fl
o
z
i
n
;
S
G
L
T
2
i
n
h
i
b
i
t
o
r
;
A
n
t
i
d
i
a
b
e
t
i
c
d
r
u
g
;
M
e
t
a
b
o
l
i
t
e
s
;
S
y
n
t
h
e
s
i
s
.
f
m
u
S
t
(
D
o
u
n
d
o
)
b
t
o
s
h
a
r
c
p
m
i
v
e
c
v
n
-
a
h
i
a
i
d
h
o
i
g
h
e
r
r
i
s
k
D
f
o
r
t
a p a g l i flo z i n (1) ( F i g
p
s
c
h
e
g
r
o
w
t
h
o
f
-
1
N
B
S
N-
B
r
o
m
o
s
u
c
c
i
n
i
m
i
d
r
e
i
c
r
1
i
v
a
i
c
u
l
a
s
o
m
p
d
i
e
l
c
n
d
e
a
t
i
o
n
e
i
s
.
A
I
B
N
A
z
o
h
b
i
s
i
s
o
b
u
t
y
r
o
n
i
t
i
l
e
r
q
v
e
u
e
s
C
s
d
i
o
a
e
r
e
d
e
n
h
d
t
v
e
d
g
c
l
o
e
d
a
s
l
b
p
y
B
r
t
i
s
t
o
n
a
s
l
d
n
e
-
M
y
e
o
v
e
r
s
L
i
O
H
.
H
2
O
L
i
t
i
u
m
h
y
d
r
o
x
i
d
e
m
o
m
o
h
y
d
r
a
t
e
b
o
d
m
y
,
s
n
n
t
r
fi
e
a
o
t
e
n
o
a
s
l
r
e
e
c
-
r
.
P
T
S
A
p
-
T
o
l
u
e
n
M et h a n o l
e
s
u
l
p
h
o
n
i
c
a
c
i
d
i
S
o
)
i
u
d
e
p
l
u
o
b
e
c
g
-
t
r
s
p
t
e
M
e
O
H
G
L
a
T
g
2
i
n
z
h
b
i
t
o
r
w
i
c
e
d
u
e
e
s
r
o
t
o
e
d
l
u
c
o
l
e
n
l
s
B
F
.
O
E
t
B
o
r
o
n
t
r
i
fl
u
r ie t h y l s i l a n e
o
r
i
d
e
d
i
e
t
h
y
l
e
t
h
e
r
a
t
e
3
2
a
p
l
i
fl
o
i
n
i
s
s
l
d
u
n
d
h
b
r
a
n
d
a
m
e
E
t
S
i
H
T
3
F
A
R
X
I
G
.
M
n
O
M
a
n
g
a
n
e
s
e
d
i
o
x
i
d
e
2
A
d
r
u
g
m
e
t
a
b
o
l
i
t
e
i
s
g
e
n
e
r
a
t
e
d
i
n
t
h
e
b
s
o
d
y
a
s
a
N
a
B
H
S
o
d
i
u
m
b
o
r
o
h
y
d
r
i
d
e
4
b
y
p
r
o
d
u
c
t
b
y
a
t
h
e
b
i
o
t
r
a
n
s
f
o
r
m
a
t
i
o
n
r
e
a
c
t
i
o
n
o
f
d
r
u
g
s
.
B
B
r
B
o
r
o
n
t
r
i
b
r
o
m
i
d
e
3
I
n
g
e
n
e
r
a
l
,
c
t
i
v
e
m
e
t
a
b
o
l
i
t
e
s
a
n
d
t
h
e
i
r
p
a
r
e
n
t
d
r
u
g
s
D
H
C
B
M
r
D
H
i
c
h
l
o
r
o
y d r o b r o m i c a c i d
m
e
t
h
a
n
e
h
a
v
e
s
i
m
i
l
a
r
b
i
o
c
h
e
m
i
c
a
l
a
n
d
p
h
a
r
m
a
c
o
l
o
g
i
c
a
l
a
c
t
i
o
n
s
.
N
e
v
e
r
t
h
e
l
e
s
s
,
p
h
e
a
r
m
a
c
o
l
o
g
i
c
a
l
t
a
c
t
i
o
n
a
n
d
t
h
e
r
a
p
e
u
t
i
c
e
f
f
e
c
b
a
t
s
o
f
a
c
f
t
i
v
m
e
t
a
b
o
l
i
t
e
s
h
o
s
e
e
a
r y
ct i v e m et a b o l i t e
e
s
n
e
r
g
i
s
t
i
c
o
r
i
n
h
i
i
t
o
r
y
o
t
h
e
p
a
r
e
n
t
d
r
u
g
s
.
T
h
a
s
a
r
e
l
s
o
h
a
v
i
n
g
a
n
i
m
p
e
r
a
t
i
v
e
I
r
o
l
e
t
o
u
n
d
e
r
s
t
a
n
d
t
h
e
e
1. Introduction
m
m
e
c
h
a
n
i
s
m
o
f
a
c
t
i
o
n
e
o
f
d
r
u
g
s
.
n
d
r
u
g
d
i
s
c
o
v
e
r
y
,
a
c
t
i
v
e
t
a
b
o
l
i
t
e
s
a
r
e
e
f
f
c
t
i
v
e
l
y
u
s
e
f
u
l
a
s
l
e
a
d
c
a
n
d
i
d
a
t
e
s
T
y
r
a
g
p
o
b
a
e
n
e
r
-
i
t
2
c
e
d
d
s
y
i
a
b
e
e
a
c
g
t
s
h
l
e
e
a
y
s
i
w
a
a
s
a
l
h
o
w
z
)
n
g
o
d
a
-
r
t
l
b
u
e
d
r
w
y
e
m
i
a
d
m
e
t
p
a
r
b
e
o
v
a
l
l
a
l
a
i
l
l
t
c
e
y
i
d
i
s
e
i
d
o
.
g
e
r
d
T
e
y
b
i
r
p
l
e
a
e
o
n
n
-
o
c
d
2
d
y
d
u
r
i
n
g
t
h
e
p
e
r
i
o
d
o
f
l
e
a
d
o
p
t
i
m
i
z
a
t
i
o
n
p
h
a
s
e
.
M
o
r
e
o
v
e
r
,
c
h
i
s
i
t
d
e
n
c
t
h
e
r
e
a
r
e
v
a
r
i
o
u
s
a
c
t
i
v
e
m e
a
t
a
b
o
l
i
t
e
s
t
h
a
t
a
r
e
a
l
r
e
a
d
y
o f e x h i b i t -
d
i
i
p
s
e
r
c
c
e
t
e
r
i
e
c
b
n
o
a
r
m
h
h
fi
e
s
t
a
b
l
i
s
h
e
d
a
s
d
r
u
g
s
a
s
c
o
n
s
e
q
u
e
n
c
e
s
u
(
h
r
m
i
a
s
b
y
r
e
v
e
c
i
n
i
n
s
u
l
i
n
s
e
c
r
e
t
i
o
n
,
a
l
o
n
g
w
i
t
h
r
e
s
i
s
t
a
n
c
e
t
o
i
n
s
u
l
i
n
,
i
n
g
s
u
p
e
r
i
o
r
p
h
a
r
m
a
c
o
l
o
g
i
c
a
l
,
p
h
a
r
m
a
c
o
d
y
n
a
m
i
c
,
*
F
o
r
c
o
r
r
e
s
p
o
n
d
e
n
c
e
supplementary material, which is available to authorized users.
4
2
P
a
g
e
2
o
f
8
J. Chem. Sci. ( 2 0 2 0 ) 1 3 2 : 4 2
a n i n t e r n a l s t a n d a r d i n D M S O - d6, D O a n d C D O D s o l -
2 3
Cl
O
CH3
1
v
e
n
t
s
.
T
h
e
H
e
c
h
e
m
i
c
a
l
s
h
i
f
t
v
a
l
u
e
s
w
e
r
e
r
e
p
o
r
t
e
d
i
n
t
h
e
d
1
3
O
s
c
a
l
e
r
e
l
a
t
i
v
t
o
T
n
M
S
(d
i
0
.
0
0
)
a
n
d
t h e
- d6,
C
c
h
e
m
i
c
a
l
s
h
i
f
t
HO
HO
v
a
l
u
e
s
w
e
r
e
g
i
v
e
r
d
p
e
l
a
t
v
e
t
o
D
M
S
O
D
O
a
n
d
C
D
O D
3
2
a
s
i
n
t
e
r
n
a
l
s
t
a
n
d
a
r
s
.
H
R
M
S
(
H
i
g
h
-
r
e
s
o
l
u
t
i
o
n
m
a
s
s
s
p
e
c
-
OH
t
r
a
l
)
a
n
a
l
y
s
i
s
w
a
s
e
r
f
o
r
m
e
d
u
s
i
n
g
e
l
e
c
t
r
o
s
p
r
a
y
i
o
n
i
z
a
t
i
o
n
OH
(
E
S
I
)
m
e
t
h
o
d
o
r
n
X
e
v
o
G
2
Q
T
O
F
m
a
s
s
s
p
e
c
t
r
o
m
e
t
e
r
.
S
p
e
c
i
fi
c
o
p
t
i
c
a
l
o
t
a
t
i
o
n
w
a
s
r
e
c
o
r
d
e
d
u
s
i
n
g
a
n
A
n
t
o
n
P
a
a
r
Figure 1.
C
h
e
m
i
c
a
l
s
t
r
u
c
t
u
r
e
o
f
d
a
p
a
g
l
i
fl
o
z
i
n
(
1
)
.
M
C
P
5
0
0
i
n
s
t
r
u
m
e
n
t
.
A
l
l
t
h
e
c
h
e
m
i
c
a
l
s
w
e
r
e
p
u
r
c
h
a
s
e
d
y
f
r o
u r i fic a t i o n
m
c
o
m
m
e
r
c
i
a
l
s
u
p
p
l
i
e
r
s
a
n
d
u
s
e
d
w
i
t
h
o
u
t
a
n
p
.
i
p
m
a n
in vivo
m
p
e
r
n
o
t
b
o
v
d
o
d
e
r
d
u
p
h
.
a
I
r
n
v
r
m
c
a
l
l
u
l
o
e
a
c
o
i
o
p
g
y
h
o
k
c
u
i
a
n
l
n
e
s
d
r
t
t
e
i
u
c
a
n
d
,
d
d
y
o
fl
p
s
a
e
h
s
f
e
n
f
g
t
t
y
p
c
r
a
a
o
fi
o
l
n
e
,
e
s
o
t
n
t
f
o
a
c
a
r
p
2
e
a
e
t
c
a
n
u
a
)
h
e
v
s
e
i
r
e
e
a
r
g
s
i
n
d
i
e
s
i
d
i
fi
t
i
t
i
2
.
2
P
r
e
p
a
r
a
t
i
o
n
o
f
(
2
S
,
3
R
,
4
R
,
5
S
,
6
R
)
-
2
-
(
4
-
c
h
l
o
r
o
-
e
d
t
a
l
i
t
e
s
o
i
s
i
a
t
a
s
t
r
t
t
g
a
f
n
s
t
a
n
y
t
e
i
z
o
a
e
e
i
e
u
w
h
n
d
m
n
e
b
a
l
u
c
g
g l
a r e
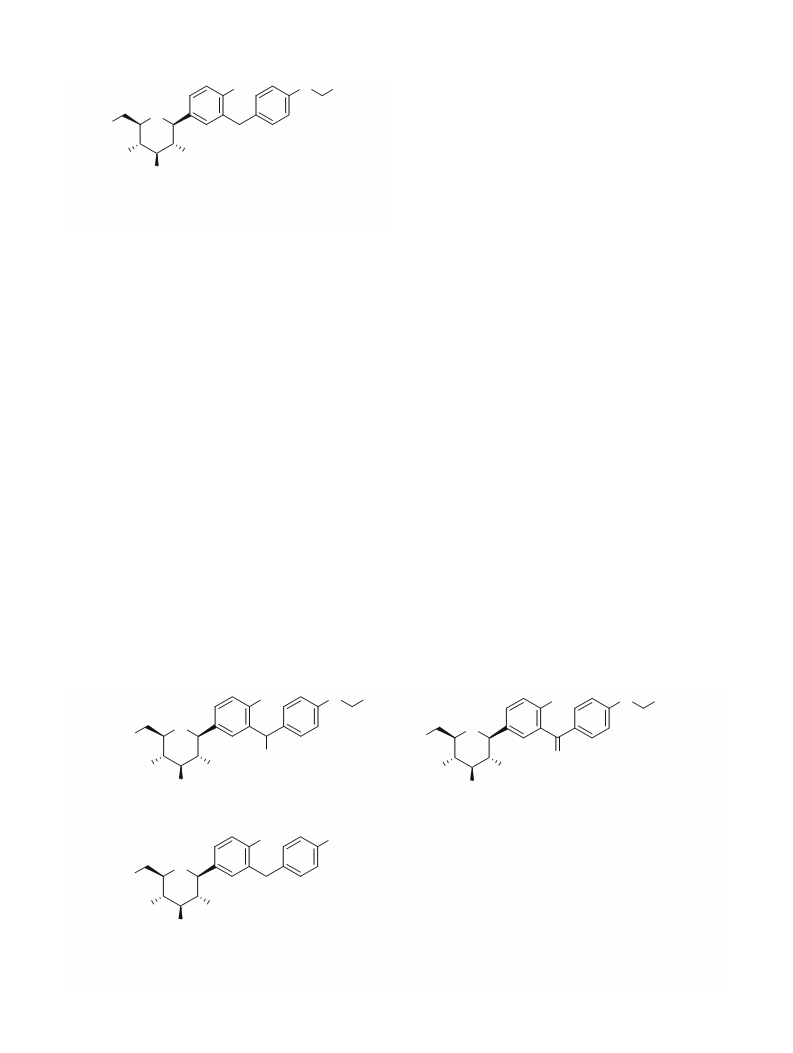
3-((4-ethoxyphenyl)(hydroxy)methyl)phenyl-6-
(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol
(benzylic hydroxy dapagliflozin) (2) (Scheme 1)
t
i
p
y
s
c
v
m
h
d
e
a
y
e
r
i
o
s
c
a
p
o
a
l
u
d
n
h
e
r
e
d
x
h
d
d
2
h
a
r
c
o
o
x
t
b
i
c
a
l
a
p
a
i
v
i
t
s
x
i
b
i
t
e
y
.
B
e
n
n
z
( 3
e
l
i
c
d
r
d
d
e
n
a
o
a
l
i
fl
i
n
(
2
)
,
o
F
.
o
d
a
i
-
fl
d
l
b
o
z
i
c
o
z
)
a
n
d
e
s
y
l
t
o
p
g
d
d
o
l
i
z
g
t
(2
i
n
(4
o
o
i s
s 2, 3
)
(
n
i g
3– 5
u
r
T
e
h
w
b
d 4
T
o
a
s
t
i
r
r
e
d
i
s
o
l
u
t
i
o
n
l
o
f
t
e
t
r
a
a
c
e
t
y
l
d
a
p
a
g
l
i
fl
o
z
i
n
( 5
(
)
(
1
5
g
,
i
s
l
o
s
,
l
d
c
c
a
s
m
c
r
I
e
t
l
i
s
a
l
a
i
fl
z
i
i
s
o
o
b
r
-
f
0
0
.
0
2
m
o
l
)
n
C
d N
H
C
(
3
5
0
m
L
)
u
n
d
e
e
r
N
, A
2
I
B
N
0
.
4
2
g
,
3
a
t
i
n
y
h
a
r
a
d
t
o
n
e
r
i
z
a
t
d
d
i
a
m
b
)
d
l
i
c
p
a
t
c
a
h
y
d
.
0
0
2
m
o
l
)
a
n
-
b
r
o
m
o
s
u
c
c
i
n
i
m
i
d
(
7
.
4
g
,
0
a
.
0
4
m
o
l
)
w
e
r
e
e
n
i
h
y
x
a
y
d
g
n
i
fl
z
m
i
n
d
e
s
r
n
i
y
e
a
d
d
e
d
.
T
h
e
m
i
x
t
u
r
e
w
a
s
w
a
r
m
e
d
a
n
d
s
t
i
r
r
e
d
t
g
e
n
t
l
e
r
e
fl
u
x
6
S
e
e
d
e
t
a
l
.
i
t
i
,
c
o
p
o
u
n
a
f
o
r
1
6
h
.
T
h
e
s
o
l
u
t
i
o
n
w
a
s
c
o
o
l
e
d
t
o
a
m
b
i
e
n
t
t
e
m
p
e
r
a
t
u
r
e
l
a
g
m
i
n
a
s
t
o
i
d
e
t
o
d
h
n
e
a
s
l
t
i
m
p
a
u
r
r
i
i
t
e
i
e
r
i
s
e
fi
q
o
f
r
d
a
p
P
a
r
e
I
g
l
i
r
b
fl
o
z
i
n
b
d
r
o
u
g
g
i
p
r
l
h
o
i
o
e
d
n
u
u
v
c
e
t
s
i
n
-
e
t
a
n
d
H
O
(
1
5
0
m
L
)
w
a
s
a
d
d
e
d
.
T
h
e
a
r
e
s
u
l
t
a
n
t
s
l
u
r
r
y
w
a
s
2
7
e
r
i
t
e
r
t
u
p
o
t
.
i
o
t
o
i
o
l
c
a
t
i
s
t
i
r
r
e
d
f
o
r
1
6
h
a
t
a
m
b
i
e
n
t
t
e
m
p
e
r
t
u
r
e
.
T
h
e
p
s
a
r
t
i
t
i
o
n
e
d
t
a
e
,
h
e
s
e
s
e
e
g
i
h
n
c
a
n
t
m
y
t
n
s
a
o
l
i
i
t
e
s 2– 4
o
s
l
d
b
o
o
r g
0
a
n
i
c
l
a
y
e
r
w
a
s
w
a
s
h
e
d
w
i
t
h
s
a
t
u
r
a
t
e
d
a
q
u
e
o
u
N a H C O
3
a
v
a
i
l
a
b
l
n
o
u
a
n
o
t
i
t
.
e
t
h
s
e
c
n
s
t
e
x t
f 2, 3
,
w
r
e
p
n
r
d 4
(
1
0
m
L
)
a
n
d
H
O
(
1
0
0
m
L
)
.
T
h
e
o
r
g
a
n
i
c
l
a
y
e
r
w
a
s
c
o
n
-
2
c
e
n
t
r
a
t
e
d
u
n
d
e
r
r
e
d
u
c
e
d
p
r
e
s
s
u
r
e
t
o
a
f
f
o
r
d
t
e
t
r
a
a
c
e
t
y
l
t
h
s
i
m
p
l
e
m
t
d
s
f
r
t
h
y
n
t
h
s
i
o
a
b
e
n
z
y
l
i
c
h
y
i
d
r
o
x
y
d
a
p
a
g
l
i
fl
o
z
i
n
(
6
)
(
1
6
g
,
1
0
0
%
)
.
u
s
i
n
g
c
o
m
m
e
r
c
i
a
l
l
y
a
c
c
e
s
s
i
b
l
e
r
a
w
m
a
t
e
r
i
a
l
s
.
T
o
a
s
t
r
r
e
d
s
o
l
u
t
i
o
n
o
f
t
e
t
r
a
a
c
e
t
y
l
b
e
n
z
y
l
i
c
h
y
d
r
o
x
y
d
a
p
a
g
l
i
fl
o
z
i
n
(
6
)
(
1
5
g
,
0
.
0
2
5
m
o
l
)
i
n
1
:
1
M e O H / H O
2
(
r
1
0
0
m
L
)
w
a
s
a
d
d
e
d
L
i
O
H
.
H
O
(
1
.
2
8
g
,
0
–
.
0
3
m
o
l
)
.
T
h
e
2. Experimental
2
e
a
c
t
i
o
n
m
i
x
t
u
r
e
w
a
s
s
t
i
r
r
e
d
f
o
r
1
6
h
a
t
2
0
3
0 °
r
C
a
n
d
t
h
e
s
o
l
v
e
n
t
w
a
s
e
v
a
p
o
r
a
t
e
d
u
n
d
e
r
r
e
d
u
c
e
d
p
r
e
s
s
u
e
.
T
h
e
r
e
s
i
-
2
.
1
G
e
n
e
r
a
l
e
x
p
e
r
i
m
e
n
t
a
l
m
e
t
h
o
d
s
d
u
e
,
a
f
t
e
r
d
i
s
s
o
l
u
t
i
o
n
i
n
M
T
B
E
(
3
0
0
m
L
)
,
w
a
s
s
u
b
s
e
q
u
e
n
t
l
y
1
1
3
w
a
s
h
e
d
w
i
t
h
c
H
O
(
7
5
m
L
)
a
n
d
b
r
i
n
e
(
7
5
m
L
)
.
T
h
e
o
a nd t h
r
g
a
n
i
c
e
H
a
n
d
C
a
N
M
R
s
p
e
c
t
r
a
w
e
r
e
r
e
c
o
r
d
e
d
o
n
B
r
u
k
e
r
A
v
a
n
c
e
2
l
a
y
e
r
w
a
s
o
n
c
e
n
t
r
a
t
e
d
u
n
d
e
r
r
e
d
u
c
e
d
p
r
e
s
s
u
r
e
3
0
0
M
H
z
n
d
V
a
r
i
a
n
5
0
0
M
H
z
s
p
e
c
t
r
o
m
e
t
e
r
u
s
i
n
g
T
M
S
a
s
Cl
O
CH3
Cl
O
O
CH3
O
O
HO
HO
HO
HO
OH
Cl
OH
OH
OH
2
OH
3
OH
O
HO
HO
OH
OH
4
Figure 2.
C
h
e
m
i
c
a
l
s
t
r
u
c
t
u
r
e
s
o
f
b
e
n
z
y
l
i
c
h
y
d
r
o
x
y
d
a
p
a
g
l
i
fl
o
z
i
n
(
2
)
,
o
x
o
d
a
p
a
g
l
i
fl
o
z
i
n
(
3
)
a
n
d
d
e
s
e
t
h
y
l
d
a
p
a
g
l
i
fl
o
z
i
n
(
4
)
.

J. Chem. Sci.
(
2
0
2
0
)
1
3
2
:
4
2
P
a
g
e
3
o
f
8
4
2
Cl
O
CH3
Cl
O
CH3
i) NBS / AIBN / CHCl3
Reflux, 16 h
O
O
AcO
AcO
AcO
AcO
OH
OAc
OAc
ii) H2O, 20-30 °C, 16 h
OAc
5
OAc
6
LiOH.H2O/
MeOH / THF / H2O
20-30 °C, 16 h
Cl
O
CH3
Cl
O
CH3
NBS / AIBN / CHCl3
Reflux, 16 h
O
O
HO
HO
HO
HO
OH
OH
OH
OH
2
OH
1
Scheme 1.
S
y
n
t
h
e
s
i
s
o
f
b
e
n
z
y
l
i
c
h
y
d
r
o
x
y
d
a
p
a
g
l
i
fl
o
z
i
n
(
2
)
.
r
e
s
i
d
u
e
w
a
s
p
u
r
i
fi
e
d
b
y
c
o
l
u
m
n
c
h
r
o
m
a
)
t
o
g
r
a
p
h
y
t
o
a
f
f
o
r
d
h
e
x
a
n
e
s
,
1
6
e
m
L
,
0
.
2
n -
5
6
m
o
l
o -
)
w
a
s
a
d
d
e
d
w
h
i
l
e
k
e
e
p
i
n
g
t
h
e
p
u
r
e
b
e
n
z
y
l
i
c
h
y
d
r
o
x
y
d
a
p
a
g
l
i
fl
o
z
i
n
(
2
(
1
.
7
0
g
,
1
5
%
=
)
0
,
a
s
a
t
e
m
p r
- O
e
a
t
u
r
b
e
t
w
e
e
7
5
t
8
0 °
l
C
.
A
f r
( 10
t
e
3
)
0
m
i
n
,
2
,
3
,
4
,
6
-
2
D
0
g
l
a
s
s
y
o
f
f
-
w
h
i
t
e
a
m
o
r
p
h
o
u
s
s
o
l
i
d
.
[
a
]
: ?
1
1
.
9 °
1
(
c
.
2
i
n
t
e
t
r
a
-
t
r
i
m
e
t
h
y
l
s
i
l
y
l
-
b
-
D
-
g
l
u
c
o
a
c
t
o
n
e
(
1
3
.
3
g
,
0
.
2
5
6
g
1
m
e
t
h
a
n
o
l
)
;
H
N
M
R
(
D
O
)
(
5
0
0
M
H
z
)
:
.
3
5
–
.
3
8
(
t
3
2
H
)
,
m
o
e
l
)
s
o
l
u
t
i
o
n
(
n
i
n
t
t -
o
l
u
e
n
e
o -
(
5
4
m
L
)
w
a
s
a
d
d
e
d
b
y
m
a
i
n
t
a
i
n
i
n
2
3
4
.
.
5
3
7
–
3
4
.
.
6
3
4
4
(
m
,
2
H
)
, 3
, J
.
7
8
–
3
.
9
2
(
m
,
2
H
)
,
4
.
1
1
–
4
.
0
9
(
m
,
H
)
,
t
h
r
e
a
c
t
i
o
a
7
5
t
8
0
°
C
.
A
f
t
e
r
6
0
m
i
n
,
m
e
t
h
a
n
e
s
u
l
-
)
6
–
(
d
,
1
H
=
9
.
5
H
z
)
,
6
.
2
, J
0
(
s
,
1
H
)
,
6
.
9
9
–
6
.
9
7
9
;
f
o
n
i
c
a
c
i
d
4
.
9
g
,
0
.
4
0
6
m
o
l
)
s
o
l
u
t
i
o
n
i
n
M
e
O
H
(
5
4
m
L
(
(
d
,
2
H
, J
,
=
9
H
z
)
,
4
)
7
.
3
5
–
7
.
3
4
(
d
,
2
H
=
8
.
5
H
z ,
, J
)
7
.
4
1
–
7
.
3
w
a
s
a
d
d
e
d
;
w
h
e
r
e
u
p
o
n
,
t
h
e
r
e
a
c
t
i
o
n
w
a
s
a
l
l
o
w
e
d
s
l
o
w
l
y
t
o
n
m
1
,
1
H
)
7
.
4
8
–
7
O
7
.
6
(
d
d
,
1
H
)
,
7
.
7
3
–
7
.
7
2
(
d
,
1
H
=
2
.
5
H
z
)
2
q
0
–
3
0
°
C
a
n
d
s
t
i
r
r
e
a
d
f
o
r
1
6
h
.
T
h
e
r
e
a
c
t
i
o
n
w
a
s
t
h
e
3
C
N
M
R
(
D
(
3
0
0
M
H
z
)
:
1
6
.
7
4
,
2
8
.
7
7
,
5
1
.
5
8
,
6
1
3
3
.
.
.
6
9
,
,
,
u
e
n
c
h
e
d
w
i
t
h
s
a
t
u
r
t
e
d
a
q
u
e
o
u
s
N
a
H
C
O
(
3
9
0
m
L
)
.
A
f
t
e
r
2
6
1
1
7
.
2
2
6
,
7
2
.
5
4
,
4
.
5
1
,
7
7
.
0
1
,
8
0
.
0
8
,
8
1
2
.
8
5
,
8
4
.
1
2
,
,
1
7
4
6
2
2
e
x
t
r
a
c
t
i
o
n
w
i
t
h
E
t
O
A
c
(
9
0
m
L
)
,
t
h
e
o
r
g
a
n
i
c
l
a
y
e
r
w
a
s
2
3
9
.
5
,
1
2
9
.
8
8
,
1
3
1
.
0
5
,
1
3
1
.
7
2
,
1
3
.
7
9
,
1
3
2
.
7
2
1
9
w
p
a
s
h
e
d
w
i
t
h
b
r
i
n
e
p
r
i
o
r
t
o
c
o
n
c
e
n
t
r
a
t
i
o
n
u
n
d
e
r
( 11
r
e
d
u
c
e
d
7
.
1
0
,
1
3
9
.
9
4
,
1
4
3
.
5
4
,
1
6
0
.
5
0
;
M
A
S
S
(
E
S
I
)
f
o
r
C
H
1
C
l
O
r
e
s
s
u
r
e
t
o
a
f
f
o
r
d
m
e
t
h
o
x
y
o
x
o
d
a
p
a
g
l
i
fl
o
z
i
n
)
(
3
y
.
5
g
,
2
2
5
7
?
(
M
-
H
)
4
2
3
.
1
2
1
0
,
f
o
u
n
d
:
4
2
3
.
1
2
0
0
;
3
5 % ) .
T
o
a
s
t
i
r
r
e
d -
( 11
4
0
t
o -
g
4
5
°
C
s
o
l
u
t
i
o
n
o
f
m
e
t
h
o
x
0
(
o
x
o
d
a
p
a
g
l
i
fl
o
z
i
n
)
(
3
.
5
,
0
.
0
1
m
o
l
)
i
n
C
H
C
l
(
2
m
L
)
,
,
2
2
E
t
S
i
H
(
3
.
0
g
,
0
e
.
0
2
7
m
o
l
)
f
o
l
l
o
w
e
d
b
y
B
F
Á
O
E
t
3
.
8
g
3
3
2
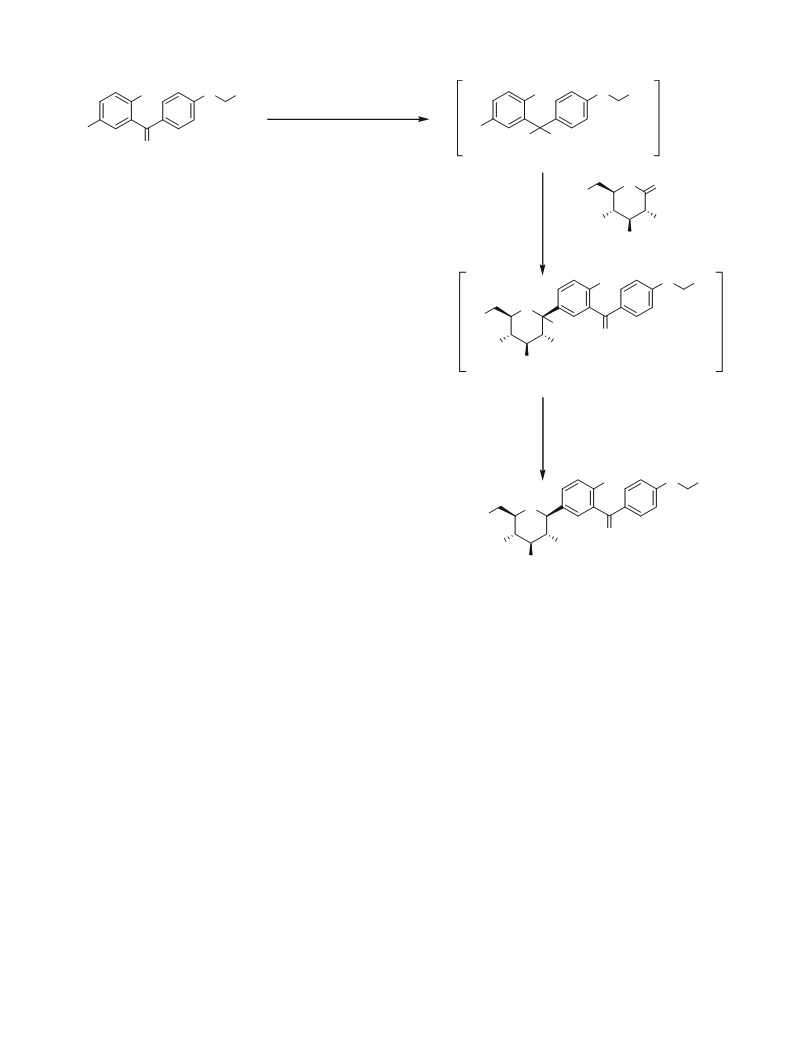
2 . 3 Preparation of (2-chloro-5-
((2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-
0
p
.
0
2
7
m
o
l
)
w
e
r
a
d
d
e
d
b
y
m
d -
a
i
n
t
a
i
n
i
n
g
t
h
e
r
e
a
c
t
i
o
n
t
e
m
-
e
r
a
t
u
r
e
b
e
t
w
e
e
n -
o
4
0
a
n
4
5 °
a
C
.
T
h
e
s
o
l
u
t
i
o
n
w
a
s
(hydroxymethyl)-tetrahydro-2H-pyran-2-
yl)phenyl)ethoxyphenyl)methanone (oxo
dapagliflozin) (3) (Scheme 2)
a
l
l
o
w
e
d
t
o
w
a
r
m
t
0
°
C
o
v
e
r
2
h
n
d
s
t
i
r
r
e
d
f
o
r
2
h
,
p
r
i
o
r
t
o
q
u
e
n
c
h
i
n
g
w
i
t
h
s
a
t
u
r
a
t
e
d
a
q
u
e o u s N a H CO ( 3 5 m L ) . A f t er
3
r
e
m
o
v
a
l
o
f
s
o
l
v
e
n
t
u
n
d
e
r
r
e
d
u
c
e
d
p
r
e
s
s
u
r
e
,
t
h
e
r
e
s
i
d
u
e
w
a
s
p
a
r
t
i
t
i
o
n
e
d
b
e
t
w
e
e
n
o
E
t
O
A
c
(
3
5
m
L
)
a
n
d
H
O
(
3
5
m
L
)
.
2
T
o
a
s
t
i
r
r
e
d
s
o
l
u
t
i
o
n
o
f
5
-
b
r
o
m
o
-
2
-
c
h
l
o
r
o
-
4 0
-
e
t
h
o
x
y
b
e
n
-
F
o
l
l
o
w
i
n
g
e
x
t
r
a
c
t
i
n
o
f
t
h
e
a
q
u
l
e
a
o
u
s
l
a
y
e
r
w
i
t
h
E
t
O
A
c
z
o
p
h
e
n
o
n
e
(
8
)
(
1
0
g
,
0
.
0
3
m
o
l
)
i
n
M
e
O
H
(
1
0
0
m
L
)
,
P
T
S
A
(
3
5
m
L
)
,
t
h
e
c
o
m
b
i
n
e
d
o
(
r
g
a
n
i
c
y
e
r
s
w
e
r
e
w
a
s
h
e
d
w
i
t
h
(
3
g
,
0
.
0
1
m
o
l
)
(
w
a
s
a
d
d
e
d
.
T
o
t
h
i
s
r
e
a
c
t
i
o
n
m
a
s
s
,
t
r
i
m
e
t
h
y
-
H
O
(
1
0
m
L
)
a
n
d
b
r
i
n
e
1
0
m
L
)
.
T
h
e
o
r
g
a
n
i
c
p
h
a
s
e
w
a
s
s
2
l
o
r
t
h
o
f
o
r
m
a
t
e
5
0
m
L
)
w
a
s
a
d
d
e
d
p
o
t
i
o
a
n
w
i
s
e
o
v
e
r
2
0
m
i
n
.
r
-
c
o
n
c
e
n
t
r
a
t
e
d
u
n
d
e
r
r
e
d
u
c
e
d
p
r
e
s
s
u
r
e
a
n
d
t
h
e
r
e
s
i
d
u
e
w
a
T
h
e
m
i
x
t
u
r
e
w
a
s
w
a
r
m
e
d
a
n
d
s
t
i
r
r
e
d
t
a
t
g
e
n
t
l
e
r
e
fl
u
x
f
o
p
u
r
i
fi
e
d
n (3)
b
y
c
o
l
u
m
n
c
h
r
o
m
a
t
o
g
r
a
p
h
y
t
o
y
i
e
l
d
o
x
o
d
a
p
a
g
l
i
-
2
4
h
.
T
h
e
m
i
x
t
u
r
c
d
e
w
a
s
c
o
o
l
e
d
t
o
a
m
b
i
e
n
t
e
m
p
e
r
a
t
u
r
e
s
u
b
fl
o
z
2
D
i
(
2
.
6
g
,
8
0
%
)
a
s
a
,
n
o
f
f
-
w
h
i
t
e
a
m
o
r
p
h
o
u
s
s
o
l
i
d
.
0
1
s
s
e
q
u
e
n
t
l
y
E
t
O
A
(
3
0
0
m
L
)
a
n
d
s
a
t
u
r
a
t
e
d
a
q
u
e
o
u
s
N
a
H
C
O
[
a
]
: ?
M
9
.
4
°
(
c
=
0
.
2
i
n
m
e
t
h
a
n
o
l
)
;
H
N
M
R
(
D
O
)
3
2
o
l
u
t
i
o
n
w
e
r
e
a
d
e
d
.
T
h
e
s
e
p
a
r
a
t
e
d
o
r
g
a
n
i
c
l
a
y
e
r
w
a
s
w
a
s
h
e
d
d
(
5
0
0
H
z
)
:
1
.
4
4
–
1
.
3
9
(
t
3
H
)
,
3
.
2
5
–
3
0
,
.
4
8
4 .1 7 ( m ,
(
m
,
4
H
)
,
,
w
i
t
h
b
r
i
n
e
(
3
0
m
L
d 9
)
,
p
r
i
o
r
t
o
t
h
e
c
o
n
c
e
n
t
r
a
t
i
o
n
u
n
d
e
r
r
e
d
u
c
e
3
4
.
.
6
1
7
8
–
–
3
4
.
9
2
0
1
(
m
,
2
H
) ,
, J
4
.
1
0
–
4
.
2
1
(
m
,
3
H
)
,
4
.
1
–
2
H
)
p
r
e
s
s
u
r
e
t
o
y
i
e
l
(
9
g
)
o -
a
s
a
w
h
i
t
e
s
o
l
i
d
.
.
(
d
,
1
H
=
9
.
3
H
z
)
,
6
.
9
8
–
7
.
0
1
(
m
2
H
)
,
7
.
4
7
,
6
–
7
.
4
4
4
T
o
a
s
t
i
r
r
e
d -
l
7
5
t
8
0 °
L
C
u
s
o
l
u
t
i
o
n
o
, n
f 9
-
(
9
g
,
0
.
2
3
3
m
o
l
)
(
(
m
m
,
1
H
)
,
7
.
4
9
(
s
,
1
H
)
,
7
.
5
5
–
7
.
5
9
(
d
d
,
1
H
)
,
7
.
6
–
7
.
7
1
3
i
n
1
:
4
T
H
F
/
t
o
u
e
n
e
(
9
0
m
)
n
d
e
r
N
B
u
L
i
(
1
5
%
w
/
v
i
n
,
2
H
)
;
C
N
M
R
(
C
D
O
D
)
(
3
0
0
M
H
z
)
:
1
4
.
4
4
1
4
.
9
4
,
2
3
4
2
P
a
g
e
4
o
f
8
J. Chem. Sci. ( 2 0 2 0 ) 1 3 2 : 4 2
Trimethylorthoformate
/ PTSA / MeOH
Cl
O
CH3
Cl
O
CH3
Br
Br
Reflux, 24 h
H3CO
OCH3
O
8
9
O
O
(i) n-Butyllithium / THF / Toluene
TMSO
TMSO
-75 to -80 °C, 30 min
OTMS
(ii) MsOH / MeOH
20-30 °C, 16 h
OTMS
10
Cl
O
CH3
O
HO
OCH3
OH
O
HO
OH
11
BF3.OEt2 / Et3SiH / DCM
0 °C, 2 h
Cl
O
O
CH3
O
HO
HO
OH
OH
3
Scheme 2.
P
r
e
p
a
r
a
t
i
o
n
o
f
o
x
o
d
a
p
a
g
l
i
fl
o
z
i
n
(
3
)
.
2
8
1
0
2
3
.
.
8
0
9
,
,
3
0
.
8
7
,
6
1
.
5
1
,
6
2
,
.
8
7
,
6
5
.
0
3
,
7
1
.
5
6
,
7
6
.
4
3
,
7
9
.
5
9
1
9
,
,
c
o
n
c
e
n
t
r
a
t
e
d
.
T
h
e
r
e
s
i
d
u
e
w
a
s
t
h
e
n
t
r
e
a
t
e
d
w
i
t
h
E
t
O
H
0
4
8
2
.
1
5
,
1
1
5
.
4
9
,
1
2
9
.
1
1
,
1
3
0
.
1
9
,
1
3
0
.
4
6
,
1
3
0
.
(
2
0
m
L
)
a
n
d
t
h
e
p
r
e
c
i
p
i
t
a
t
e
d
s
a
l
t
s
w
e
r
e
r
e
m
o
v
e
d
b
y
c
e
l
i
t
e
nd er r ed uc ed
1
.
7
,
1
3
3
.
6
9
,
1
3
9
.
6
3
1
4
0
.
2
3
,
1
6
5
.
2
6
,
1
7
2
.
9
9
,
1
M ?
9
5
.
7
5
;
:
b
p
e
d
fi
l
t
r
a
t
i
o
n
.
T
h
e
fi
l
t
r
a
t
e
w
a
s
c
o
n
c
e
n
t
r
a
t
e
d
y
fl
u
?
H
R
M
S
(
E
S
I
)
c
a
l
c
u
l
a
t
e
d
f
o
r
C
H
C
l O (
7
H
)
r
e
s
s
u
r
e
a
n
d
t
h
e
r
e
s
i
d
u
e
w
a
s
p
u
r
i
fi
e
d
b
c
o
l
u
m
n
c
h
r
o
-
2
1
2
3
4
2
3
.
1
2
1
0
,
f
o
u
n
d
:
4
2
3
.
1
1
9
8
;
m
a
t
o
g
r
a
p
h
y
t
o
a
f
f
o
r
d
p
u
r
e
d
e
s
e
t
h
y
l
d
a
p
a
g
l
i
o
z
i
n
(4
5°
) ( 1. 6 g )
( c = 0 .2
2
0
a
s
a
g
l
a
s
s
y
o
f
f
-
w
h
i
t
e
a
m
o
r
p
h
o
u
s
s
o
l
i
d
.
[
a
]
:
?
5
.
D
1
i
n
m
e
t
h
a
n
o
l
)
;
H
N
M
R
( D O) ( 50 0 M Hz ): 3 .4 6– 3. 59 ( m,
2
4
J
6
H
)
,
3
.
7
3
&
3
.
8
4
(
2
d
d
,
2
H
)
,
3
.
9
5
(
s
,
2
H
)
,
4
.
1
5
–
4
.
1
8
(
d
,
1
, J
H
,
2
. 4 Preparation of (2S,3R,4R,5S,6R)-2-(4-chloro-
3-(4-hydroxybenzyl)phenyl)-6-
=
9
.
3
H
z
)
,
6
.
7
4
–
6
.
7
6
(
d
,
2
H
, J
, J
, J
=
6
3
H
z
)
,
7
.
0
5
–
6
7
.
0
7
(
d
,
2
H
=
H
z
)
,
7
.
2
2
–
7
.
2
3
(
d
,
1
H
=
H
z
)
,
7
.
2
(
s
,
1
H
)
,
7
.
3
0
(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol
(desethyl dapagliflozin) (4)
(
s
,
1
H
)
,
7
.
3
7
–
7
.
4
0
(
d
,
1
H
=
9
H
z
)
;
H
,
R
M
S
(
E
S
I
)
c
a
l
c
u
l
a
t
e
d
?
f
o
r
C
H
C
1
lO ( M-
6
H
)
:
3
7
9
.
0
9
4
8
f
o
u
n
d
:
3
7
9
.
0
9
3
6
;
1
9
2
A
H
m
i
x
t
u
r
e
o
f
d
a
p
a
g
l
i
fl
o
z
i
n
( 1
%
)
(
5
g
,
0
.
0
1
2
m
o
l
)
a
n
d
a
q
u
e
o
u
s
d
d
B
r
(
r
1
2
.
6
g
,
0
.
0
7
3
m
o
l
,
4
8
w
/
w
)
w
e
r
e
w
a
r
m
e
d
a
n
d
s
s
t
i
r
r
e
2 . 5 Preparation of tetraacetyl oxodapagliflozin
(7) (Scheme 3)
u
n
d
e
a
g
e
n
t
l
e
r
e
fl
u
x
f
o
r
1
6
h
.
T
d
h
e
r
e
a
c
t
i
o
n
m
a
s
s
w
a
c
o
o
l
e
t
o
a
n
a
m
b
i
e
n
t
t
e
m
p
e
r
a
t
u
r
e
a
n
p
a
r
t
i
t
i
o
n
e
d
b
e
t
w
n
e
e
n
M
T
B
E
(
5
0
m
L
)
a
n
d
H
O
(
5
0
m
0
L
m
u
)
.
F
o
l
l
o
w
i
n
g
e
x
t
r
a
c
t
i
o
o
f
a
c
q
u
e
o
u
s
s
2
T
o
a
s
t
i
r
r
e
d
s
o
l
u
t
i
o
n
o
f
t
e
t
r
a
a
c
e
t
y
l
d
a
p
a
g
l
i
fl
o
z
i
n
(
5
)
(
1
0
g
,
l
a
y
e
r
w
i
t
h
M
T
B
E
(
5
L
)
,
t
h
e
c
o
m
b
i
n
e
d
o
r
g
a
n
i
l
a
y
e
r
0
.
0
1
m
o
l
)
i
n
C
H
C
l
(
1
0
0
0
m
L
)
i
,
a
c
t
i
v
a
t
e
d
M
n
O
(
1
5
0
g
,
w
e
r
e
n
e
u
t
r
a
l
i
z
e
d
w
i
t
h
a
q
e
o
u
s
N
a
O
H
s
o
l
u
t
i
o
n
.
T
h
e
o
r
g
a
n
i
c
3
2
1
.
7
2
m
o
l
)
w
a
s
a
d
d
e
d
.
T
h
e
r
e
a
c
t
o
n
m
a
s
s
w
a
s
w
a
r
m
e
d
a
n
d
l
a
y
e
r
w
a
s
s
e
p
a
r
a
t
e
d
,
w
a
s
h
e
d
w
i
t
h
H
O
(
5
0
m
L
)
a
n
d
2
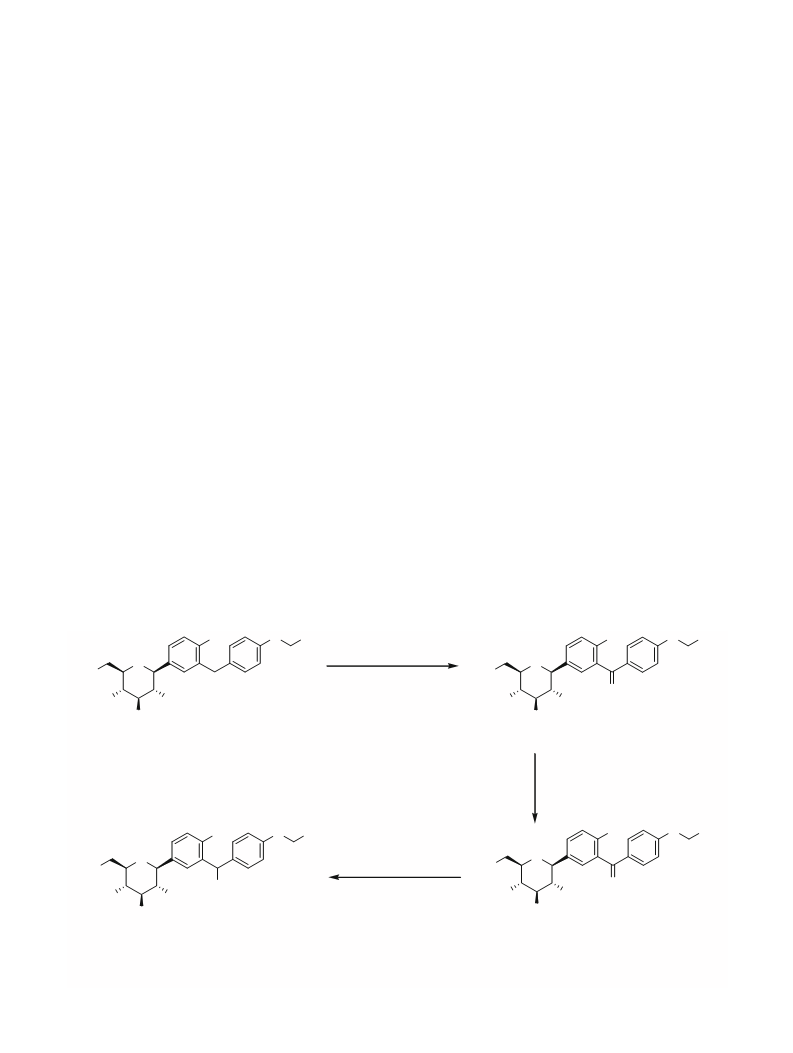
J. Chem. Sci.
(
2
0
2
0
)
1
3
2
:
4
2
P
a
g
e
5
o
f
8
4
2
s
t
i
r
r
e
d
u
n
d
e
r
a
m
c
g
e
n
t
l
e
r
e
fl
u
x
f
o
r
1
e
2
.
h
.
T
h
e
r
e
a
c
t
i
o
n
m
a
s
s
w
a
s
2
.
7 Preparation of benzylic hydroxy dapagliflozin
(2) (Scheme 3)
c
o
o
l
e
d
t
o
a
b
i
e
n
t
t
e
m
p
e
r
a
t
u
r
a
n
d
t
h
e
i
n
s
o
l
u
b
l
e
s
w
e
r
e
r
e
m
o
v
e
d
b
y
e
l
i
t
e
b
d
e
d
fi
l
t
r
a
t
i
o
n
T
h
e
fi
l
t
r
a
t
e
w
a
s
c
o
n
c
e
n
-
t
l
r
a
t
e
d
u
n
d
e
r
r
e
d
u
c
e
p
r
e
s
s
u
r
e
,
a
n
d
t
h
e
r
e
s
i
d
u
e
w
a
s
c
r
y
s
t
a
l
-
T
o
a
s
t
i
r
r
e
d
0
°
C
s
o
l
u
t
i
o
n
o
f
o
x
o
d
a
p
a
g
l
i
fl
o
z
i
n
0
(
3
)
(
2
g
,
i
z
e
d
f
r
o
m
a
m
i
x
t
u
r
e
o
f
M
e
O
H
(
1
0
0
m
L
)
a
n
d
C
H
C
l
0
.
0
0
4
m
o
e
l
)
i
n
M
e
O
H
(
1
0
m
L
)
,
N
a
B
H
(
0
.
2
g
,
.
0
0
4
m
o
l
)
s
s
3
4
(
2
0
m
L
)
t
o
a
f
f
o
r
d
t
e
t
r
a
a
c
e
t
y
l
o
x
o
d
a
p
a
g
l
i
fl o
-d6
z
i
n
( 7
0
)
(
8
.
3
g
,
w
w
a
a
s
a
d
d
d
p
o
r
t
i
o
n
-
w
i
s
e
o
v
e
r
2
0
m
i
n
.
T
h
e
m
i
x
t
u
r
e
w
a
1
8
1
1
1
%
)
a
s
a
w
h
i
t
e
s
, J
o
l
i
d
.
H
N
M
R
(
D
M
S
O
)
(
3
0
M
H
z
)
:
r
m
e
d
t
t
o
2
–
0
–
3
0
°
C
a
n
d
s
t
i
r
r
e
d
f
o
r
1
6
h
.
T
h
e
m
i
x
t
u
r
e
w
a
.
3
2
–
1
.
.
3
7
(
t
,
3
H
=
6
7
H
z
)
,
2
.
4
8
(
s
,
3
H
)
,
1
.
9
3
(
s
,
3
H
)
,
c
o
o
l
e
d
o
0
5 °
w
C
a
n
d
a
c
i
d
i
fi
e
d
w
i
t
h
a
q
u
e
o
u
s
H
C
l
s
o
l
u
t
i
o
n
.
.
9
8
–
2
0
1
(
d
,
6
H
, J
,
=
.
6
H
z
)
,
4
.
0
5
–
4
.
1
7
5
(
m
,
5
H
)
,
4
.
7
5
–
4
, J
.
7
9
T
h
e
s
o
e
l
v
e
n
t
a
s
e
v
a
p
o
r
a
t
e
d
u
n
d
e
r
r
e
d
u
c
e
d
p
r
e
s
s
u
r
e
,
t
h
e
(
d
,
1
H
, J
,
=
9
.
6
H
z
)
4
.
9
6
–
5
.
1
3
(
m
,
2
H
)
,
.
3
3
–
5
.
4
0
(
t
,
1
H
=
r
(
r
e
s
i
d
u
w
a
s
p
a
r
t
i
t
i
o
n
e
d
b
e
t
w
e
e
n
M
T
B
E
(
4
0
m
L
)
a
n
d
H
O
2
9
7
2
3
7
1
1
.
4
5
H
z
)
7
.
0
3
–
7
.
0
7
(
m
1
,
3
2
H
N
)
,
7
.
4
1
(
d
,
1 , J
- d6) ( 3 0 0 M H z ) :
H
=
1
.
8
H
z
)
,
1
0
m
L
)
.
T
h
e
o
r
g
a
n
i
c
l
a
y
e
r
w
a
s
c
o
n
c
e
n
t
r
a
t
e
d
u
n
d
e
r
.
0
1
7
7
–
7
.
6
2
4
5
(
m
,
4
H
)
;
C
M
R
(
D
M
S
O
e
d
u
c
e
d
p
r
e
s
s
u
r
e
t
o
a
a
f
f
o
r
d
b
e
n
z
y
l
i
c
h
y
d
r
o
x
y
d
a
p
a
g
l
i
fl
o
z
i
n
d
0
9
4
2
6
.
.
.
0
7
2
7
5
,
0
0
.
.
2
0
3
5
,
,
2
0
.
.
3
5
3
,
,
2
6
0
2
.
.
4
6
3
,
3
8
.
.
6
7
6
6
,
3
8
.
.
9
4
8
,
,
3
7
9
2
.
.
2
3
1
5
,
,
3
7
2
6
d
9
.
.
.
.
4
9
2
7
4
,
( 2 3
( S e e a b o v e ) .
)
(
.
7
g
,
8
5
%
)
s
a
g
l
a
s
s
y
o
f
f
-
w
h
i
t
e
a
m
o
r
p
h
o
u
s
s
o
l
i
,
4
0
3
3
,
6
3
,
6
8
3
3
9
9
0
6
3
,
,
7
7
.
0
0
,
1
1
4
.
6
6
,
1
2
7
.
2
2
,
1
2
8
.
3
6
,
1
2
9
.
4
8
,
1
1
,
9
9
.
.
5
6
,
,
1
3
2
.
0
4
,
1
3
6
.
4
6
,
1
3
8
.
4
0
,
1
6
3
.
3
4
,
1
6
8
.
6
2
,
,
1
7
0
.
0
4
M ?
,
1
9
2
.
4
9
;
H
R
M
S
(
E
S
I
)
c
a
l
c
u
l
a
t
e
f
o
r
3. Results and Discussion
?
C
H
C
l
O
(
N
H
)
:
6
0
8
.
1
9
8
7
,
f
o
u
n
d
:
6
0
8
.
2
0
0
7
;
2
9
3
1
1
1
4
3
.
(2) (Scheme 1)
1
P
r
e
p
a
r
a
t
i
o
n
o
f
b
e
n
z
y
l
i
c
h
y
d
r
o
x
y
d
a
p
a
g
l
i
fl
o
z
i
n
2
(Scheme 3)
.
6
P
r
e
p
a
r
a
t
i
o
n
o
f
o
x
o
d
a
p
a
g
l
i
fl
o
z
i
n
(
3
)
T
w
W
(5
A
w
h
a
o
)
e
s
h
w
o
h
s
c
y
o
Z
h N
n
m
i
t
h
m
g
-
e
e
l
b
u
f
x
n
s
n
e
r
t
u
i
s
c
o
f
b
w
e
n
h
n
i
z
y
l
i
t
o
c
h
a
t
y
c
d
e
r
N
,
s
.
a
r
t
a
o
y
x
l
a
S
u
o
e
g
d
y
d
c
)
b
f
d
a
a
l
t
u
t
d
z
y
p
g
a
l
d
e
n
g
l
i
o
fl
z
o
i
z
n
i
n
( 5
( 2
)
)
8
e
d
i
t
t
9
e
r
a
a
p
i
fl
.
a p a g l i fl o z i n
T
o
a
s
t
i
r
r
e
d
s
o
l
u
t
i
o
n
1
o
f
t
e
t
r
a
a
c
e
t
y
l
o
x
o
d
a
p
a
g
l
i
fl
o
z
i
n
(
7
)
l
–
e
r
o
y
r
y
y
r
e
a
c
t
i
o
f
i
I
y
n
y
z
d
e
t
e
t
y
(
6
g
,
0
.
0
1
m
o
l
)
i
n
(
:
2
:
3
H
O
/
T
H
F
/
M
A
e
O
H
(
7
2
m
L
)
w a s
w a s
w a s
w a s
e
2
i
t
m
o
s
i
u
c
c
n
i
m
d
e
(
)
e
)
B
s
s
h
a
y
d
h
i
n
h
e
r
p
t
a
r
e
s
e
n
a
l
c
m
b
t
w
o
e
o
f
a
d
d
e
d
L
i
O
H
.
H
O
h
0
.
5
g
,
0
.
0
1
2
m
o
l
)
.
f
t
e
r
t
h
e
m
i
x
t
u
r
e
2
z
b
i
s
i
s
O
d
b
o
b
r
o
n
t
r
i
l
e
(
A
B
N
s
e
q
t
r
e
t
e
n
t
s
t
i
r
r
e
d
f
o
r
1
6
a
r
t
2
0
–
3
0
°
C
,
t
h
e
r
e
a
c
t
i
o
n
m
a
s
s
i
t
H
h
n
i
s
h
e
d
t
h
e
o
r
b
s
n
t
h
i
T
p
h
t
e
a
c
s
e
t
y
e
n
-
2
c
o
n
c
e
n
t
r
a
t
e
d
u
n
d
e
r
e
d
u
c
e
d
p
r
e
s
s
u
r
e
.
T
h
e
r
e
s
i
d
u
e
z
a
y
c
l
e
i
c
y
r
o
d
a
p
a
g
l
y
d
l
i
fl
z
o
e
h
i
(
6
h
y
r
o
l
y
i
s
o
)
g
( 2
f
e
t
r
a
p
o
r
t
i
o
n
e
d
b
e
t
w
e
e
n
6
0
m
L
e
a
c
h
o
f
E
t
O
A
c
a
n
d
a
H
O . T h
2
t
y
l
e
z
g
r
c
l
i
c
h
d
x
n
y
d
l
i
fl
o
i
n
d
o
( 6
a
i
t
h
n
o
r
g
a
n
i
c
l
a
y
e
r
w
a
s
w
a
s
h
e
d
w
i
t
h
H
O
(
3
0
m
L
)
n
d
c
o
n
c
e
n
-
)
2
L
(2
i
O
H
.
H
e
d
O
e
n
e
r
n
a
t
e
y
l
i
c
r
p
.
o
x
a
z
g
p
i
l
i
fl
)
i
z
i
2
t
r
a
t
e
d
u
n
d
e
r
r
e
d
u
c
e
d
p
e
r
e
s
s
u
r
e
t
o
a
f
f
o
r
d
o
d
x
o
d
a
p
a
g
l
i
fl
o
z
i
n
(
3
)
o
.
T
h
p
y
u
e
o
b
e
z
y
i
c
r
o
x
r
y
a
a
y
a
g
l
i
fl
r
n
w
a
s
a
a
s
a
o v e) .
g
l
a
s
s
y
o
f
f
-
w
h
i
t
a
m
o
r
p
h
o
u
s
s
o
l
i
(
3
.
6
g
,
8
4
%
)
(
s
e
e
i
s
l
a
t
e
b
l
u
m
n
c
h
r
o
m
a
t
o
g
p
D
u
i
n
t
h
e
n
i
t
i
a
l
b
Cl
O
CH3
Cl
O
CH3
MnO2 / CHCl3
Reflux, 16 h
O
O
AcO
AcO
AcO
AcO
O
OAc
OAc
OAc
5
OAc
7
LiOH.H2O /
MeOH / THF / H2O
20-30 °C, 16 h
Cl
O
CH3
Cl
O
CH3
O
O
HO
HO
NaBH4 / MeOH
20-30 °C, 16 h
HO
HO
O
OH
OH
OH
OH
3
OH
2
Scheme 3.
A
l
t
e
r
n
a
t
i
v
e
s
y
n
t
h
e
s
i
s
o
f
b
e
n
z
y
l
i
c
h
y
d
r
o
x
y
d
a
p
a
g
l
i
fl
o
z
i
n
(
2
)
a
n
d
o
x
o
d
a
p
a
g
l
i
fl
o
z
i
n
(
3
)
.
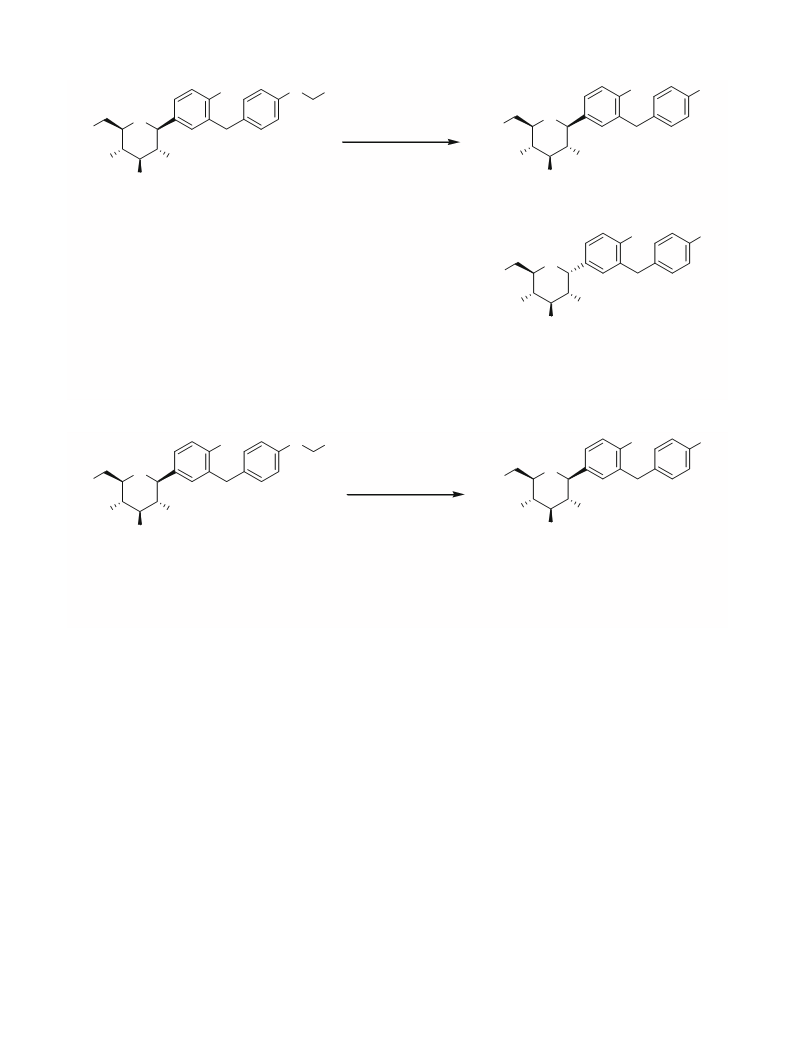
4
2
P
a
g
e
6
o
f
8
J. Chem. Sci. ( 2 0 2 0 ) 1 3 2 : 4 2
Cl
OH
OH
Cl
O
CH3
O
O
HO
HO
BBr3 / DCM
HO
HO
OH
OH
OH
OH
4
OH
1
+
Cl
O
HO
HO
OH
4a
Scheme 4.
R
e
p
o
r
t
e
d
s
y
n
t
h
e
s
i
s
o
f
d
e
s
e
t
h
y
l
d
a
p
a
g
l
i
fl
o
z
i
n
(
4
)
.
Cl
OH
Cl
O
CH3
O
O
HO
HO
Aqueous HBr
Reflux, 16h
HO
HO
OH
OH
OH
4
OH
1
Scheme 5.
P
r
o
p
o
s
e
d
s
y
n
t
h
e
s
i
s
o
f
d
e
s
e
t
h
y
l
d
a
p
a
g
l
i
fl
o
i
z
i
n
(
4
)
.
d
5
e
w
v
e
a
l
s
o
p
u
m
e
u
n
c
t
c
,
a
n
s
a
t
l
t
.
e
m
p
t
t
o
p
r
e
p
a
r
e
2
f
r
o
m
1
i
n
s
t
e
a
d
o
f
R
11
e
d
u
u
c
s
t
i
v
g
e
e
l
i
m
t
i
n
a
l
t
o
a
n
n
o
f
m
d
e
t
B
h
F
o
x
.
u
y
O
c
o
E
t
x
t
w
o
d
a
p
r
o
a
d
l
g
e
a
l
d
t
i
fl
o
o
z
x
b
i
n
o
y
n
s
e
s
f
u
i
n
t
r
i
e
h
y
s
i
l
e
a
n
a
s
f
f
o
3
2
d
a
p
a
g
l
i
fl
o
z
i
n
(
3
)
.
T
h
e
p
u
r
e
p
r
o
d
a
i
s
e
d
c
m
4 0
o
e
l
u
n
t
m
n
c
a
h
t
i
r
t
p
o
m
p
e
a
t
t
y
o
t
l
g
o
m
r
p
a
p
h
p
a
y
a
n
.
D
u
e 3
r
u
i
n
g
n
s
o
g
u
5
d
r
-
i
n
o
i
m
o
t
i
a
o
l
-
d
e
c
v
h
e
l
o
r
p
o
-
-
3
. 2 Preparation of oxo dapagliflozin (3)
(Scheme 2)
t
,
a
n
e
m
r
e
r
s
i
b
r
2
-
l
o
8
-
n s u c ce s s fu l .
e
h
o
x
y
d
h
n
e
t
h
e
i
n
t
e
a
f
8
w
a
s
u
T
w
T
h
e
h
e
s
y
5
r
n
t
h
e
m
s
i
s
o
n
o
f
-
o
x
o
r
5
d
a
p
-
o
a
e
m
g
l
i
o
fl
x
o
y
-
z
b
c
i
n
e
l
o
d
(
3
)
p
-
w
h
40
a
s
o
i
n
n
e
x
i
i
t
i
(8
b
a
t
)
e
.
n
d
8
i
t
-
b
r
o
-
2
c h
f
l
o
o
-
40
t
h
n
z
o
e
n
h
e
a
c
t
i
o
o
-
b
r
o
-
2
h
o
r
o
-
e
t
h
o
0
y
n
e
t
-
3 3 Alternative synthesis of benzylic hydroxy
dapagliflozin (2) and oxo dapagliflozin (3)
.
1
z
o
p
h
e
e
n
n
c
o
e
n
o
e
(8
f p
)
w
i
e
t
n
h
e
t
r
i
l
m
e
o
t
h
n
y
l
o
a
r
c
t
h
i
f
o
r
m
a
t
e
h
e
( P T S A ) a f f o r d e d
p
r
e
s
-
t
o
l
u
s
u
p
h
i
c
c
e
h
n
o
r
o
r
e
x
g
e
e
u
s
y
e
n
p
p
n
t
y
e
m
o
h
n
e
e
d
n
x
l
i
y
c
i
n
l
h
g
)
a
i
-b-D
c
x
k
m
e
e
t
t
o
a
h x
f 9
l
o
i
.
e
.
e
4
-
)
e
b
b
d
r
e
o
n
b
m
o
n
-
1
e
d
,
-
c
( 9
i
,
h
l
)
i
o
.
o
-
r
L
n
t
o
-
2
h
f
r
-
i
(
u
t
(
4
m
e
-
I
a
b
fl
n
p
e
o
e
n
r
s
p
o
y
n
i
a
t
e
c
o
f
s
t
h
w
r
e
o
r
a
e
f
a
e
y
w
c
t
t
h
o
p
a
i
t
a
m
a
t
t
i
l
h
e
n
o
s
o
q
n
a
g
z
b
o
e
v
n
( 2
t
o
e
e
o
p
r
h
a
r
o
p
o
s
e
d
s
o
a
r
u
s
y
t
a
v
c
t
n
h
p
e
t
e
h
s
a
s
e
i
g
y
o
w
t
z
l
n
n
i
c
e
-
-
s
t
h
d
n
a
i
y
m
t
h
y
l
z
e
i
t
p
r
h
e
e
r
p
r
s
i
u
)
g
t
o
y
n
a
a
l
o
g
e
,
o
f
m
a
o
l
l
o
w
y
a
2
d
3
g
t
4
n
o
t
t
h
n
z
l
i
c
h
,
y
d
o
r
u
x
e
d
a
v
h
a
s
o
e
g
i
fl
i
n
n
a
h
o
d
l
o
x
d
i
s
c
t
h
t
e
d
a
c
h
d
r
a
t
i
c
t o
( 10
,
6
e
a
a
-
O
-
z
i
(
3
)
t
h
c
o
n
r
e
y
t
r
a
a
n
i
n
f
o
t
e
r
n
t
i
8
t
r
i
m
t
h
l
s
o
e
i
l
y
l
-
g
l
u
o
l
c
t
o
n
e
)
e
e
r
d in situ
e
d
a
t
h
s
d
i
s
t
h
a
t
c
l
d
a
t
n
d
c
h
m
t
c
g
.
r
a
p
i
c
p
u
q
i
fi
a
t
i
m
a
i
x
o
t
r
f
l
a
t
o
l
s
,
x
w
i
a
c
h
w
e
r
e
d
i
e
s
i l
(11
y
l
)
a
t
e
t
o
a
p
l
o
e
s
n
e
g
d
t
h
a
i
e
s
y
n
r
i
c
s
s
u
t
e
C
n
s
e
h e t i c p a t h w ay t o r e so l v
e
n
l
y
,
e
e
f
f
r
d
t
h
o
y
o
o
p
a
g
l
i
fl
o
z
n
b
y
t
r
e
a
t
m
e
n
t
o
p
n
l
t
e
a
t
i
v
e
w
i
t
h
m
e
t
h
a
n
e
s
u
l
p
h
o
n
i
c
a
c
i
d
(
M
s
O
H
)
i
n
m
e
t
h
a
n
o
l
.
t
h
e
a
f
o
r
e
m
e
n
t
i
o
n
e
d
i
s
s
u
e
s
(
S
c
h
e
m
e
3
)
.
J. Chem. Sci.
(
2
0
2
0
)
1
3
2
:
4
2
P
a
g
e
7
o
f
8
4
2
T
e s
d 3
h
i
e
m
)
e
p
u
f
r
l
r
o
p
n
m
b
o
e
s
o
t
g
e
u
e
a
d
s
t
n
c
s
o
y
a
n
n
c
c
t
e
h
t
t
o
a
t
n
n
t
i
h
s
e
e
s
y
s
d
n
o
a
o
t
t
p
u
t
h
f
a
x
l
l
g
e
y
o
e
t
g
i
t
h
l
d
d
o
i
o
i
c
e
fl
a
p
p
o
r
o
m
( 7
a
c
e
)
h
p
e
l
h
r
i
e
d
m
i
s
o
t
t
e
( 2
a
l
d
Acknowledgements
t
a
s
m
M
T
b
T
w
S
d
b
h
n
y
t
a
s
l
i
t
w
t
a
b
o
t
e
W
L
e
a
r
e
g
r
a
t
e
f
u
l
t
o
t
h
e
m
a
n
a
g
e
m
e
n
t
o
f
A
u
r
o
b
i
n
d
o
P
h
a
r
m
a
-
o
r
a
t
y
p
i
a
o
z
n
i
n
.
T
e
t
t
y
l
-
i
m
i
t
e
d
W
.
,
f
o
r
t
h
e
g
e
n
e
r
o
u
s
s
u
p
p
o
r
t
a
n
d
c
o
n
s
t
a
n
t
e
n
c
o
u
r
a
g
e
n
t
h
a
s
i
s
e
u
a
w
i
t
t
h e
a c
a c
t
i
o
o
a p a g l i fl o z i n ( 5) w i t h
f
t
h
i
a
r
m
e
n
t
.
e
a
r
e
a
l
s
o
t
h
a
n
k
f
u
l
t
o
t
h
e
c
h
e
m
i
c
a
l
a
n
d
a
n
a
l
y
t
i
c
a
l
e
t
h
n
1
e
1
g
o
r
o
p
i
n
t
e
r
r
x
a
a
e
e
a
t
y
y
a
r
r
e
s
e
a
r
c
h
d
e
p
a
r
t
m
e
n
t
s
f
o
r
t
h
e
i
r
c
o
-
o
p
e
r
a
t
i
o
n
.
n
O
t
f
f
o
r
c
o
d
t
e
x
f
o
z
M
o
d
a
p
( 7
a
g
)
l
w
a
g
i
a
n
fl
o
z
i
C
i
s
n
(
7
)
e
.
d
.
)
.
2
h
e
p
r
h
u
y
y
L
q
l
l
r
e
t
e
t
r
a
a
i
s
e
t
y
l
o
d
l
fl
o
i
n
s
d
o
l
a
C
t
y
c
s
t
a
l
l
i
z
a
s
t
i
n
o
i
n
t
m
a
e
i
x
e
O
d
p
e
H
p
a
H
l
3
References
h
e
d
i
u
r
O
e
o
o
l
y
f
e
e
o
n
r
a
c
e
t
l
o
o
k
x
a
a
g
g
l
i
fl
o
o
z
z
n
i
i
n
( 7
( 3
o
i
t
h
H
t
.
H
O
u
)
x
g
i
i
d
r
f
e
l
a
t
e
d
x
d
a
n
f
l
i
fl
u
H
i
)
o
d
2
1
.
M
M
e
a
m
D
n
n
g
o
o
W
,
a
B
P
M
r
,
,
u
c
R
c
H
e
a
o
,
A
v
t
E
,
r
,
a
A
l
e
x
G
a
,
r
J
n
d
G
r
a
A
g
N
,
,
a
T
P
P
t
e
h
P
M
g
i
g
y
p
,
J
M
A
M
S
,
,
u
b
s
e
g
y
n
r
n
d
e
d
(3
c
,
t
o
d
p
i
r
o
a
e
z
r
s
i
y
e
n
l
n
e
t
o
r
o
p
n
i el d e
x
r
n
a
m
i
t
n
d
a
B
h
N
R
a
n
P
p
W
s
R
l
i
a
e
p
n
a
z
i
fl
z
h
i
y
h
a
c
(2
e
)
o
N
a
B
y
4
E
a
n
P
S
A
o
M
b
e
t
o
Z
,
r
a
h
n
,
D
a r y T O ,
n
n
i
e
i
c
r
o
y
a
p
g
i
fl
.
P
,
e
b
o
r
a
h
L
N
a
t
n
,
s
e
h
W
W
M
i
,
l
S
W
i
c
t
a
s
l
i
o
a
m
G
H
g
W
,
H
i
t
A
,
s
J
h
o
m
i
h
s
n
h
K
R
,
W
L
,
2
o
r
E
e
v
0
r
l
l
a
D
,
J
J
a
,
o
s
m
O
v
e
l
e
i
s
i
G
R
P
f
,
A
i
y
i
n
g
n
g
p
i
n
n
B
v
e
r
F
,
J
e
a
n
a
n
d
a
l
l
i
a
N
e
r
i
W
c
0
e
S
8
D
i
l
s
c
r
y
o
d
p
a
p
a
g
d
l
i
-
t
e
3
.
4
P
r
e
p
a
r
a
t
i
o
n
o
f
d
e
s
e
t
h
y
l
d
a
p
a
g
l
i
fl
o
z
i
n
(
4
)
fl
o
z
n
o
m
:
s
p
o
e
n
t
,
s
l
e
t
i
v
e
n
a
o
d
u
b
m
-
d
e
e
n
r
e
t
n
h
g
l
u
e
c
o
t
r
a
n
s
p
o
2
r
t
e
2
(
e
G
L
T
2
)
i
n
h
s J. Med. Chem. 51
i
i
t
o
r
f
o
t
r
e
a
r
e
n
t
o
f
t
y
R
p
e
-
e
o
d
f
e
a
b
e
t
1
1
4
5
e t a bo -
P
r
i
o
r
t
o
o
u
r
s
y
n
t
h
e
s
i
s
,
t
w
o
s
y
n
t
h
e
t
i
c
a
p
p
r
o
a
c
h
e
s
w
e
r
e
2
.
.
F
u
A
n
2
d
0
0
6
o
l
o
p
h
a
r
m
a
d
c
o
e
l
e
o
g
i
c
a
l
e
l
y
a i
t Drug Discov.
c
t
v
e
m
r
fl
e
o
p
z
e
o
i
s
r
n
t
e
(1
i
d
f
)
b
u
o
u
r
s
i
m
d
e
g
o
t
h
e
s
e
B
f
r
t
h
B
y
r
e
l
d
(
a
S
p
c
s
d
n
a
h
y
s
g
e
n
a
l
m
t
i
fl
e
e
o
z
4
c
e
i
)
n
.
r
c
u
e
(
4
)
f
W
r
o
e
i
e
d
S
m
e
n
d
e
c
d
a
l
p
u
a
a
g
l
i
-
d
o
1
2
,
1
3
l
Today 11 1 3 3
i
t
e
i
r
u
g
d
i
s
c
v
r
y
a
n
d
v
l
o
p
m
n
i
n
v
o
a
r d e r
t
e
t
3
t
h
u
t
a
i
l
t
y
a
t
a
h
l
s
e
h
n
t
t
i
o
u
t
n
e
s
3
O
L i
W
b
e
r
W
m
,
e
K
u
r
c
s
i
e
o
n
y
r
M
,
r
Y
a
e
o
M
B
W
,
,
K
h
a
l
I
p
o
a
s
l
n
n
i
n
c
a
h
o
i
fl
n
A
,
a
K
o
l
A
h
p
a
,
l
o
S
W
w
,
h
i
t
D
a
r
z
B
,
Z
h
z
u
a
a
a
)
i
M
L
,
,
s
c
a
h
t
i
s
f
y
e
o
o
g
r
e
i
n
e
e
d
c
n
o
u
t
e
r
a
d
m
a
m
a
j
i x
4
o
r
-
)
m
o
o
s
k
i
K
E
a
y
n
B
c
u
i
s
c
e
J
n
M
a
l
l
n
f a
e
.
B
o
t
t
h
e
s
(4a
e
s
y
t
h
e
i
r
o
t
e
s
y
i
e
l
m
e
a
u
a
t
s
m
r
e
h
b
r
e
o
o
W ,
W
M
G
n
g
,
s
w
r
t
h
l
i
5
I
e
y
n
n
d
d
a
H
p
m
h
a
s
k
i
2
0
1
0
v
t
r
o
a
r
a
c
t
e
z
a
t
i
o
n
t
u
r
e
-
i
s
o
m
r
)
a
n
d
b
-
i
s
o
m
r
(
4
)
(
h
e
.
p
p
h
o
i
n
e
t
i
c
s
o
f
d
a
a
g
l
i
o
z
i
n
(
B
t
M
y
S
-
1
2
1
4
8
b
,
N
e
v
e
r
t
h
e
l
e
s
s
,
b
-
i
s
o
m
e
r
w
a
s
t
h
e
r
e
q
u
i
r
e
d
m
e
t
a
b
o
l
i
t
e
o
f
n
t
d
u
m
-
g
l
u
c
o
s
e
c
t
r
a
s
p
o
r
t
e
r
p
e
I
i
n
h
i
t
o
r
,
d
a
p
C
a
o
s
l
n
e
t
g
g
n
l
s
f
p
i
fl
o
e
z
r
t
i
i
h
n
n
n
e
e
.
g
i
T
C
T
c
g
d
J
S
X
2
s
g
T
a
B
2
n
a
n
i
m
a
l
s
a
n
d
h
u
m
a
n
s
D
r
u
g
M
e
t
a
b
.
D
i
s
p
o
s
.
3
8
4
0
5
i
d
t
y
h
n
e
l
e
t
i
s
h
d
u
n
m
m
i
t
a
o
t
i
o
f 4
n
,
s
o
t
f
w
o
p
t
a
h
s
o
e
r
n
e
d
c
u
p
o
p
m
y
t
r
u
r
t
e
e
e
n
d
s
t
e
e
p
r
l
o
e
d
c
e
-
4
.
i
r
m
o
g
t
h
F
y
,
P
R
,
M
i
c
,
h
J
a
e
n
l
J
C
G
,
a
E
i
v
W
a
,
n
J
B
J
n
,
M
T
h
W
o
m
a
d
s
E
M
D
a rk
,
d
d
u
v
v
o
a
p
m
i
s
r
e
o
r
s
t
h
i
s
i
i
i
s
a
b
t
o
a
i
F
r
a
n
c
i
s
L
i
a
,
T
e
a
a
n
2
0
n
1
4
C
a
e
r
t
c
y
i
n
o
o
f
g
d
e
n
i
c
a
i
g
t
y
r
i
s
z
k
a
,
s
a
s
n
e
s
i
s
n
m
e
b
n
i
t
t
s
r
u
o
p
f
p
s
o
o
r
t
d i u m -
s
t
h
e
e
e
a
c
n
a
p
fl
i
o
t
d
i
a
e
w
s
C
i
s
y
n
a
e
s
e
t
i
w
c
r
h
,
o
u
t
h
e
t
v
e
r
o
h
e
i
e
a
y
s
-
h
r
o
i
c
s
a
f
a
p
l
i
fl
o
i
n
h
i
o
a
d
a
g
r
e
o
s
.
e
i
d
n
s
c
a
s
o
c
i
t
e
i
t
t
e
e
r
o
c
e
v
i
s
s
o
e
s
l
d
s
c
r
l
e
n
i
b
d
l
i
a
e
u
u
c
o
s
e
P
B
e
s
c
m
o
e
-
l
t
l
r
i
a
a
t
c
,
h
i
n
n
n
u
e
R
a
o
s
p
o
r
t
e
r
2
,
i
n
s Diabetes Ther. 5
t
h
e
t
7
m
r
e
3
,
,
,
z
a
t
m
e
n
t
o
f
t
y
p
e
2
p
r
u
s
fl
e
s
o
n
s
q
y
o
e
n
t
l
y
w
f
c
u
d
o
v
a
u
t
e
a
b
e
t
e
n
o
i
l
z
y
i
e
u
5
c
n
r
t
v
e
t
h
e
d
r
t
i
c
p
p
i
y
a
r
o
t
o
o
l
( 1
r
s
f
o
d
r
e
s
t
h
l
,
-
t
h
5
6
.
.
p
e
a
d
B
5
s
n
h
a
r
m
u
t
i
c
b
a
e
L
f
i
t
l
g
,
d
i
r
S
i
e
T
e
n
t
e
r
v
Y
i
,
e
C
w
h
f
e
o
n
r
2
P
e
l
0
1
5
n
g
e
u
,
g
Y
n
m
0
5
e
d
i
o
X
l
t
i
o
g
n
.
d
a
l
(
4
)
f
r
a
l
a
g
l
fl
o
z
e
e
i
n
)
g
H
.
r
S
o
r
p
i
s
i
g
l
y
,
D
r
a
e
h
L
u
v
B
Z
a
o
g
r
J
Y
e
n
K
,
D
d
g
n
u
y
d
J
,
s
i
l
e
h
o
t
t
t
l
h
h
w
y
e
s
i
s
p
o
f
a
y
e
i
e
t
h
l
t
h
e
u
S
n
u
p
i
n
d
a
p
a
a f fo rd ed
,
J
,
n
n
i
p
t
a
n
g
X
,
e
X
d
u
b
G
e
F
y
a
n
M
-
t
1
4
0
1
e
u
C
P
h
s
P
e
p
a
n
-
m
r
g
t
a
t
a
a
c
o
h
r
a
u
t
e
y
S
r
a
t
n
n
9
-
b
z
e
n
e
e
c
e
o
n
g
d
p
l
e
u
o
r
z
i
n
r
e
s
e
n
c
o
o
b
f
q
u
o
.
m
B
e
r
i
d
v
i
n
o
b
e
l
t
o
r
e
f
f
e
c
t
o
s
o
d
i
-
d
2
p
e
n
e
d
d
d
e
e
y
l
d
i
a
p
a
g
l
e
fl
d
z
i
n
(
4
)
u
b
s
q
m
u
e
t
n
o
t
l
y
,
a
t
h
e
y
l
L
c
o
c
o
r
s
u
U
.
.
P
a
t
e
n
t
0
6
1
0
6
B
r
e
p
m
r
o
a
s
s
o
l
a
t
y
c
o
l
u
c
h
r
o
a
g
r
p
h
7 .
8 .
9 .
0 .
a
r
e
i
c
t
a
n
d
a
r
d
s
L
t
d
.
(
S
c
h
e
)
.
e
c
3
.
s
e
s
e
E
C
d
,
-
o
W
n
:
1
2
m
J
N
u
l y
W
,
,
e
2
P
0
1
9
i
L
t
h
c
c
r
0
.
u
0
S
i
l
l
i
a
h
G
i
l
p
T
M
2
S
i
, G a n g W a n d W e i M
o
a
r
y
t
l
6
g
l
u
c
5
o
,
s
i
d
S
2
n
h
i
b
i
t
r
s
a
n
d
m
e
t
h
o
d
4. Conclusions
U
D
P
a
t
e
n
,
5
1
1
1
7
B
j
e
r
a
s
s
a
i
n
C
1
9
t
4
e
8
d
B
r
m
o
m
i
n
u
a
n
t
d
i
o
n
T
s
w
i
t
h
W
N
-
–
b
Z
r
o
m
o
e
s
r
u
c
c
a
i
n
c
-
-
I
n
s
s
z
u
m
m a
f 2, 3
n
r
r
y
,
w
e
s
u
d 4
n
c
c
e
s
n
s
d
e
f
u
t
l
l
y
d
e
m
a
a
d 3
o
b
n
o
c
.
s
t
r
e
a
t
e
d
o
e
t
h
e
s
y
n
l
e
n
-
-
t
i
t
m e
io n Chem. Rev. 43 2 7
i
d
d
r
e
l
a
c
o
p
o
s
.
h
e
o
h
l
i
e
g
l
r
e
t
h
e
o
d
i
s
o
I
e
a
n
,
a
e
h
e
m
e
t
l
i
t
s
f
,
d
e
a
p
a
c
n
g
i
a
a l s o
i
n
1
1
1
1
G
f
h
o
s
a
h
t
S
a
a
n
n
d
d
G
h
n
a
u
t
a
k
t
U
R
1
9
t
8
8
n
C
a
r
s
b
o
g
n
–
t
c
a
m
r
b
e
o
n
y
b
l
o
a
n
n
d
d
fl
i
n
a
.
l
e
a
a
e
d
t
d
i
t
i
o
,
n
y
w
d
s
n
v
o
e
f 2
l
o
p
e
d
o
n
c
i
s
f
fi
o
r
m
i
o
n
a
n
l
a
i
o
n
r
e
a
c
i
o
s
u
i
n
r
i
t
h
a
n
t
c
n
v
i
v
e
e
s
e
y
t
h
e
s
i
a
n
A
s
i
i
m
p
w
l
e
e
-
t
r
i
e
100
t
h
y
l
o
r
t
h
o
f
o
r
m
a
t
e
s
P
r
o
c
.
I
n
d
i
a
A
c
a
d
.
S
c
i
.
(
C
h
e
m
.
S
c
i
)
t
d
i
o
e s c r i b e d .
s
e
l
t
i
d
t
h
l
a
t
i
o
o
f
d
a
p
a
g
l
i
fl
o
z
n
a
s
2
3
5
1
.
.
E
T
k
g
X
r
n
e
s
t
F
P
a
n
d
S
t
u
a
r
t
P
S
1
9
6
3
O
x
i
d
a
t
i
o
n
b
y
s
o
l
i
d
s
I
y
n
I
.
h
e
p
r
e
p
a
r
a
t
i
o
n
o
f
e
i
t
h
e
r
t
e
t
r
a
a
r
y
l
e
t
h
a
n
e
s
o
r
d
i
a
r
l
-
e
a
t
n
o
e
n
s
e
e
s
b
y
x
t
i
h
d
e
o
x
i
d
a
t
i
o
n
o
f
d
i
e J. Org. Chem. 28
a
r
y
6
l
m
e
t
h
a
n
e
s
w
i
t
h
m
a
Supplementary Information (SI)
d
i
o
3
8
1
1
3
H
N
M
R
,
C
N
M
R
a
n
d
H
R
M
S
s
p
e
c
t
r
a
o
f
a
l
l
c
o
m
p
o
u
n
d
s
2
u
B
,
,
X
F
u
e
n
M
g
Y
,
C
h
,
e
P
n
e
g
n
H
g
,
K
S
,
o
D
n
g
o
Y
,
L
v
Z
B
h
,
a
W
n
u
Y
,
,
W
a
a
n
n
g
g
C
,
a
r
e
a
v
a
i
l
a
b
l
e
a
t
.
L
i
S
,
D
u
J
n
g
J
,
g
W
Z
h
T
,
4
2
P
a
g
e
8
o
f
8
g
J. Chem. Sci.
(
2
0
2
0
)
1
3
2
:
4
2
Z
h
u
d
L
B
,
a
4 0
D
i
n
d
o
H
,
n
n
S
Y
a
h
e
2
p
o
n
0
g
1
t
r
Z
1
,
C
t
s
W
-
e
l
y
i
h
l
e
r
i
g
n
l
d
u
c
(
a
c
t
S
A
,
d
R
o
b
e
b
r
g
s
o
h
e
J
Y
,
1
1
3
4
.
.
C
h
e
e
d
n
Y
,
H
e
C
a
h
d
e
0
e
n
g
H
,
L
i
S
,
W
u
n
n
Y
g
d
,
F
a
e
e
n
t
n
d
h
g
Y
o
s
,
L
v
e
B
J
P
, X u B ,
9
S
e
e
n
C
h
e
A
r
o
s
i
e
s
s
u
t
i
t
u
t
e
d
S
e
B
,
d
n
M
J
e
,
r
D
u
t
J
,
W
s
a
a
C
m
R
d
b
o
e
r
g
Y
2
0
0
a
t
t
h
e
n
h
-
p
s
i
t
i
o
s
c
o
t
e
n
a
n
d
s
l
e
i
v
e
r
e
n
)
a
l
i
s
d
i
u
m
-
B
A
R
e
n
z
y
l
b
n
z
e
d
i
v
a
i
v
e
o
f
u
s
e
C
T
I
n
t
.
d
e
p
e
t
d
e
n
t
g
l
u
c
o
s
e
-
a
n
p
o
r
t
e
e
2
G
L
T
2
n
i
b
i
t
o
r
s
p
p
l
.
2
0
0
9
2
6
5
3
7
A
1
f
o
r
e
Lett. 21
t
r
e
a
t
m
e
n
t
o
f
t
y
p
e
2
d
i
a
b
t
e
s
B
i
o
o
r
g
.
M
e
d
.
C
h
e
m
.
a
n
u
B
C
a
n
d
B
h
a
r
S
1
9
9
r e v ie w Org. Prep. Proced. Int. 28 3 7 1
6
D
e
a
l
k
y
l
a
t
i
o
n
o
f
e
t
h
e
r
s
.
A
4
4
6
5

 Bonige, Kishore Babu
Bonige, Kishore Babu
 Chavakula, Ramadas
Chavakula, Ramadas
 Karumanchi, Kishore
Karumanchi, Kishore
 Korupolu, Raghu Babu
Korupolu, Raghu Babu
 Natarajan, Senthil Kumar
Natarajan, Senthil Kumar
 Peruri, Badarinadh Gupta
Peruri, Badarinadh Gupta






