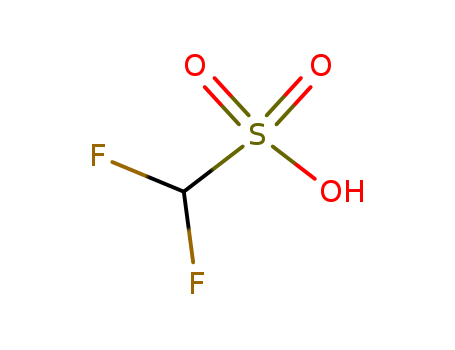
- Chemical Name:Difluoromethanesulphonic acid
- CAS No.:40856-07-3
- Molecular Formula:CH2 F2 O3 S
- Molecular Weight:132.088
- Hs Code.:
- European Community (EC) Number:255-115-0
- DSSTox Substance ID:DTXSID50193830
- Nikkaji Number:J294.772B
- Wikidata:Q72463122
- Mol file:40856-07-3.mol
Synonyms:Difluoromethanesulphonic acid;difluoromethanesulfonic acid;40856-07-3;EINECS 255-115-0;Difluoromethanesulphonicacid;CH2F2O3S;difluoromethane sulfonic acid;SCHEMBL446345;DTXSID50193830;C-H2-F2-O3-S;AKOS005063799;BB 0261613;FT-0739307