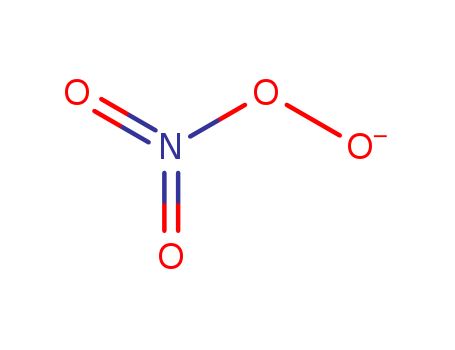
- Chemical Name:Oxido nitrate
- CAS No.:125239-87-4
- Molecular Formula:NO4
- Molecular Weight:78.0043
- Hs Code.:
- DSSTox Substance ID:DTXSID301032974
- Wikipedia:Peroxynitrate ion
- Wikidata:Q27109996
- Mol file:125239-87-4.mol
Synonyms:peroxynitrate ion;oxido nitrate;azoperoxoate;CHEBI:29270;dioxidoperoxidonitrate(1-);DTXSID301032974;[NO2(OO)](-);[NO4](-);Q27109996