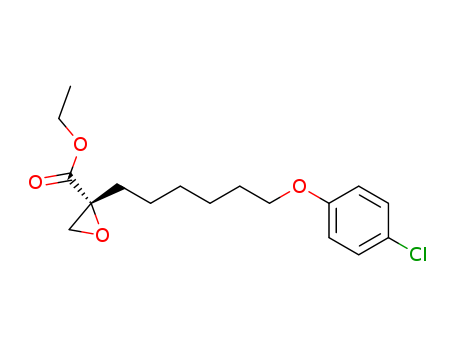
- Chemical Name:R-(+)-Etomoxir
- CAS No.:124083-20-1
- Molecular Formula:C17H23 Cl O4
- Molecular Weight:326.82
- Hs Code.:
- European Community (EC) Number:602-976-9
- UNII:MSB3DD2XP6
- DSSTox Substance ID:DTXSID801025772
- Nikkaji Number:J425.307H
- Wikipedia:Etomoxir
- Wikidata:Q27077245
- NCI Thesaurus Code:C81111
- ChEMBL ID:CHEMBL2051959
- Mol file:124083-20-1.mol
Synonyms:ethyl 2-(6-(4-chlorophenoxy)hexyl)oxirane-2-carboxylate;etomoxir