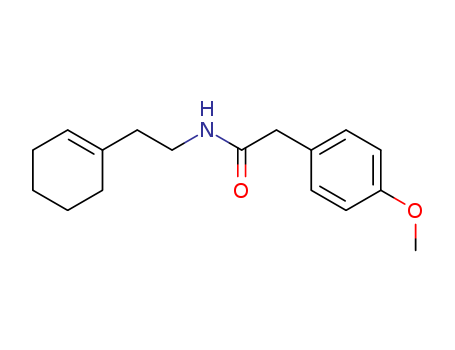
- Chemical Name:N-[2-(1-cyclohexen-1-yl)ethyl]-2-(4-methoxyphenyl)acetamide
- CAS No.:51072-34-5
- Molecular Formula:C17H23 N O2
- Molecular Weight:273.375
- Hs Code.:2924299090
- European Community (EC) Number:256-948-2
- UNII:7G8A8HNZ3J
- DSSTox Substance ID:DTXSID50199079
- Nikkaji Number:J261.626B
- Wikidata:Q83071972
- ChEMBL ID:CHEMBL1594445
- Mol file:51072-34-5.mol
Synonyms:51072-34-5;N-[2-(1-cyclohexen-1-yl)ethyl]-2-(4-methoxyphenyl)acetamide;N-[2-(cyclohexen-1-yl)ethyl]-2-(4-methoxyphenyl)acetamide;N-(2-(Cyclohex-1-en-1-yl)ethyl)-2-(4-methoxyphenyl)acetamide;7G8A8HNZ3J;N-(2-(1-Cyclohexen-1-yl)ethyl)-2-(4-methoxyphenyl)acetamide;EINECS 256-948-2;N-(2-(1-Cyclohexen-1-yl)ethyl)-4-methoxybenzeneacetamide;Benzeneacetamide, N-(2-(1-cyclohexen-1-yl)ethyl)-4-methoxy-;N-[2-(cyclohex-1-en-1-yl)ethyl]-2-(4-methoxyphenyl)acetamide;N-[2-(1-Cyclohexen-1-yl)ethyl]-4-methoxybenzeneacetamide;CBMicro_015497;UNII-7G8A8HNZ3J;Oprea1_455348;Oprea1_692129;MLS000684012;SCHEMBL6544386;CHEMBL1594445;DTXSID50199079;HMS2730C20;STK023656;AKOS000496032;NCGC00245624-01;BS-52121;SMR000291656;BIM-0015434.P001;CS-0327119;E77024;AN-329/12344844;SR-01000465851;SR-01000465851-1;N-[2-(cyclohexen-1-yl)ethyl]-p-methoxyphenylacetamide;ACETAMIDE, N-(2-(1-CYCLOHEXEN-1-YL)ETHYL)-2-(P-METHOXYPHENYL)-