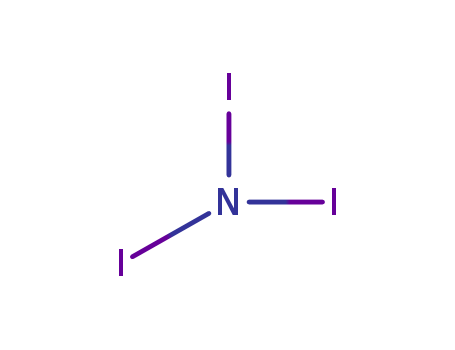
Chemical Property of Nitrogen triiodide
Edit
Chemical Property:
- Boiling Point:°Cat760mmHg
- Flash Point:°C
- PSA:3.24000
- Density:4.278g/cm3
- LogP:2.33840
- XLogP3:2.8
- Hydrogen Bond Donor Count:0
- Hydrogen Bond Acceptor Count:1
- Rotatable Bond Count:0
- Exact Mass:394.7165
- Heavy Atom Count:4
- Complexity:8
- Purity/Quality:
-
99% *data from raw suppliers
NITROGEN IODIDE 95.00% *data from reagent suppliers
Safty Information:
- Pictogram(s):
Explodes at slightest touch when dry; when handled it should be kept wet with ether. Too sensitive to be used as explosive, because it cannot be stored, handled, or transported safely.
- Hazard Codes:Explodes at slightest touch when dry; when handled it should be kept wet with ether. Too sensitive to be used as explosive, because it cannot be stored, handled, or transported safely.
- MSDS Files:
-
SDS file from LookChem
Useful:
- Chemical Classes:Other Classes -> Other Inorganic Compounds
- Canonical SMILES:N(I)(I)I
-
Description
Nitrogen triiodide (black unstable crystals) explodes at the slightest touch when dry. When handled, it is kept wet with ether. It is too sensitive to be used as an explosive, because it cannot be stored, handled, or transported.