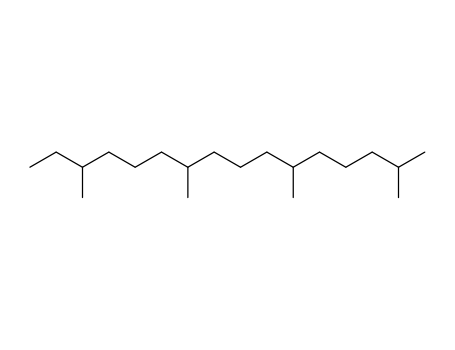
- Chemical Name:Phytane
- CAS No.:638-36-8
- Molecular Formula:C20H42
- Molecular Weight:282.553
- Hs Code.:2901100000
- European Community (EC) Number:211-332-2
- UNII:27UZX1Q8TR
- DSSTox Substance ID:DTXSID70862339
- Nikkaji Number:J12.304H
- Wikipedia:Phytane
- Wikidata:Q171701
- Metabolomics Workbench ID:28477
- Mol file:638-36-8.mol
Synonyms:phytane