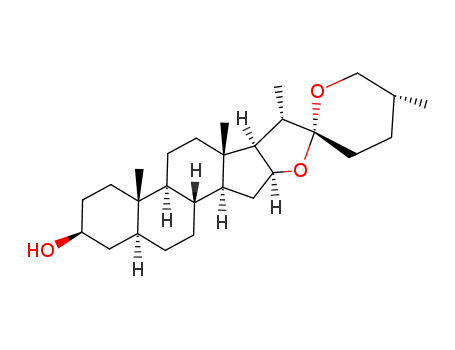
- Chemical Name:Tigogenin
- CAS No.:77-60-1
- Molecular Formula:C27H44O3
- Molecular Weight:416.645
- Hs Code.:2932999099
- European Community (EC) Number:201-041-9
- UNII:4SMU15RR44
- DSSTox Substance ID:DTXSID40903920
- Nikkaji Number:J4.209I
- Wikidata:Q27108442
- Metabolomics Workbench ID:35103
- ChEMBL ID:CHEMBL43871
- Mol file:77-60-1.mol
Synonyms:epi-sarsasapogenin;epismilagenin;PYM 50028;PYM-50028;PYM50028;sarsaponin;sarsasapogenin;sarsasapogenin, (3beta,5alpha,25R)-isomer;sarsasapogenin, (3beta,5alpha,25S)-isomer;sarsasapogenin, (3beta,5beta)-isomer;sarsasapogenin, (3beta,5beta,25R)-isomer;sarsasapogenin, (3beta,5beta,25S)-isomer;smilagenin;tigogenin