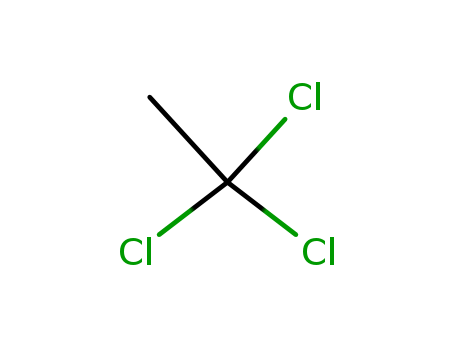
- Chemical Name:1,1,1-Trichloroethane
- CAS No.:71-55-6
- Deprecated CAS:74552-83-3
- Molecular Formula:C2H3Cl3
- Molecular Weight:133.405
- Hs Code.:2903191090
- European Community (EC) Number:200-756-3
- ICSC Number:0079
- NSC Number:9367
- UN Number:2831
- UNII:113C650IR1
- DSSTox Substance ID:DTXSID0021381
- Nikkaji Number:J2.376K
- Wikipedia:1,1,1-Trichloroethane
- Wikidata:Q161268
- NCI Thesaurus Code:C75637
- Metabolomics Workbench ID:49536
- ChEMBL ID:CHEMBL16080
- Mol file:71-55-6.mol
Synonyms:1,1,1-trichloroethane;Inhibisol;methylchloroform


 Xn,
Xn,  N,
N,  T,
T,  F
F
 Xn:Harmful;
Xn:Harmful;

