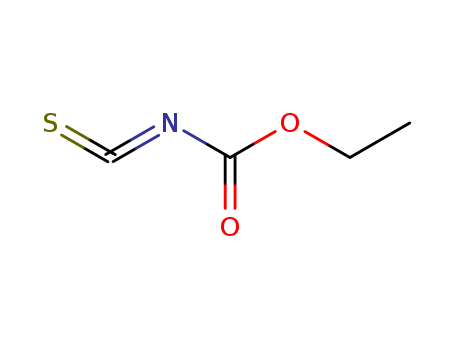
- Chemical Name:Ethoxycarbonyl isothiocyanate
- CAS No.:16182-04-0
- Molecular Formula:C4H5NO2S
- Molecular Weight:131.155
- Hs Code.:29309090
- European Community (EC) Number:240-318-9
- NSC Number:158480
- UNII:WQY39AV49E
- DSSTox Substance ID:DTXSID50167253
- Nikkaji Number:J281J
- Mol file:16182-04-0.mol
Synonyms:Ethoxycarbonyl isothiocyanate;16182-04-0;Ethyl isothiocyanatoformate;Ethoxycarbonylisothiocyanate;Carbethoxy isothiocyanate;ethyl N-(sulfanylidenemethylidene)carbamate;O-ethyl carbonisothiocyanatidate;Carbon(isothiocyanatidic) acid, ethyl ester;MFCD00004814;Carbon(isothiocyanatidic)acid Ethyl Ester;EINECS 240-318-9;ETHYL CARBONISOTHIOCYANATIDATE;NSC-158480;CARBON(ISOTHIOCYANATIDIC)ACID, ETHYL ESTER;ethyl N-carbothioylcarbamate;CARBETHOXYISOTHIOCYANATE;ethoxycarbonylisothiocyanat;ethoxycarbonyl-isothiocyanat;ethoxy carbonylisothiocyanate;ethoxycarbonyli-sothiocyanate;ethyl isothiocyanatocarbonate;ethylmethanoyl isothiocyanate;WQY39AV49E;SCHEMBL42860;ethoxy carbonyl isothiocyanate;ethoxy-carbonyl isothiocyanate;Ethyl isothiocyanatidocarbonate;Ethyl isothiocyanatidocarbonate #;DTXSID50167253;Ethoxycarbonyl isothiocyanate, 98%;AMY10862;Isothiocyanatoformic Acid Ethyl Ester;NSC158480;AKOS005198535;AB00381;NSC 158480;Carbonisothiocyanatidic acid, ethyl ester;E0440;FT-0625742;EN300-51998;A26273;J-520459;J-670007;J-801006


 T,
T,  Xi
Xi


