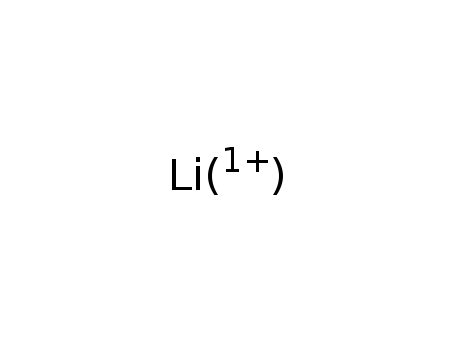- Chemical Name:Lithium ion
- CAS No.:17341-24-1
- Molecular Formula:Li
- Molecular Weight:6.941
- Hs Code.:2827391000
- UNII:8H8Z5UER66
- DSSTox Substance ID:DTXSID10169612
- Nikkaji Number:J247.008J
- Wikidata:Q66760324
- NCI Thesaurus Code:C69075
- RXCUI:1546265
- ChEMBL ID:CHEMBL1234004
- Mol file:17341-24-1.mol
Synonyms:lithium ion;lithium cation;Lithium(1+);Lithium, ion (Li1+);17341-24-1;Lithium ions;lithium, ion;Lithium(+);CHEBI:49713;UNII-8H8Z5UER66;Li(+);8H8Z5UER66;Li+;lithium(I);lithium(I) cation;Lithium (I) ion;lithium(1+) ion;Li(+) cation;Li(+) ion;Lithium, ion (Li1);LITHIUM ION CHROMATOGRAPHY STANDARD;CHEMBL1234004;DTXSID10169612;BDBM50259153;DB01356;Q66760324