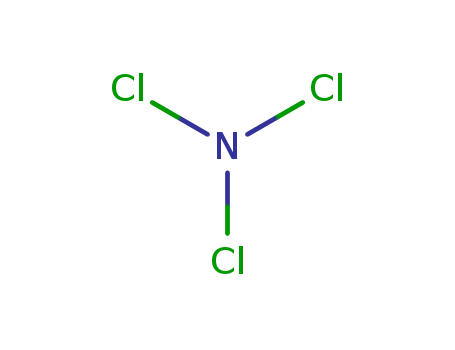
Chemical Property of Nitrogen trichloride
Edit
Chemical Property:
- Appearance/Colour:Yellow oily liquid
- Vapor Pressure:131mmHg at 25°C
- Melting Point:-40 °C (233 K)
- Boiling Point:70 °C at 760 mmHg
- PKA:-22.67±0.70(Predicted)
- Flash Point:°C
- PSA:3.24000
- Density:1.72 g/cm3
- LogP:1.74980
- Water Solubility.:insoluble H2O, decomposes in H2O after 24h; soluble CS2, phosphorus trichloride, benzene, CCl4, CHCl3 [MER06]
- XLogP3:2.6
- Hydrogen Bond Donor Count:0
- Hydrogen Bond Acceptor Count:1
- Rotatable Bond Count:0
- Exact Mass:118.909632
- Heavy Atom Count:4
- Complexity:8
- Purity/Quality:
-
99% *data from raw suppliers
TRICHLORINE NITRIDE 95.00% *data from reagent suppliers
Safty Information:
- Pictogram(s):
Explodes when heated to approximately 200F (93C) or when exposed to direct sunlight. Toxic by ingestion and inhalation, strong irritant.
- Hazard Codes:Explodes when heated to approximately 200F (93C) or when exposed to direct sunlight. Toxic by ingestion and inhalation, strong irritant.
- MSDS Files:
-
SDS file from LookChem
Useful:
- Chemical Classes:Nitrogen Compounds -> Other Nitrogen Compounds
- Canonical SMILES:N(Cl)(Cl)Cl
-
Physical properties
Yellow, oily, heavy liquid; pungent odor; density 1.653 g/mL; freezes to rhombohedral crystalline solid below -40°C; evaporates in air rapidly; vaporpressure 150 torr at 20°C; explodes when heated at 93°C; highly unstable, decomposes explosively in light; insoluble in water, decomposes slowly in cold water after several hours; decomposes in hot water; soluble in benzene, chloroform, carbon tetrachloride, carbon disulfide and phosphorus trichloride.
-
Uses
Bleaching of flour; wastage control of citrus fruit.