10.1039/b808156e
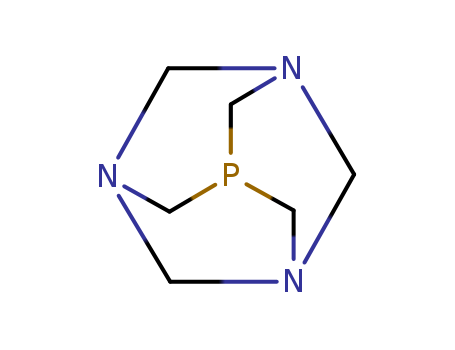
This research explores the synthesis and characterization of water-soluble azido- and tetrazolato-platinum(II) complexes using 1,3,5-triaza-7-phosphaadamantane (PTA) and its derivatives. The primary objective was to develop an environmentally friendly and efficient method for synthesizing 5-substituted-1H-tetrazoles, which have significant applications in pharmaceuticals and materials science. The researchers reacted cis-[Pt(N3)2(PPh3)2] with PTA or [Me-PTA]I to obtain diazido-platinum(II) complexes, which were then subjected to [2 + 3] cycloadditions with organonitriles under microwave irradiation to form bis(tetrazolato) complexes. The tetrazoles were easily liberated from these complexes using aqueous HCl, yielding high-purity products. The study concluded that the use of PTA in these reactions offers a convenient and efficient route for synthesizing and isolating tetrazoles, with the added advantage of water-soluble byproducts that simplify separation processes. This approach is expected to be applicable to a broader range of reactions, potentially expanding the scope of metal-mediated synthesis of nitrogen-containing heterocycles.