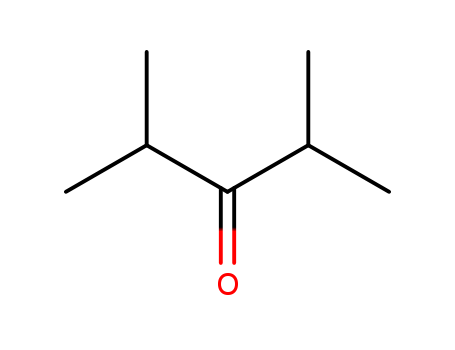
- Chemical Name:2,4-Dimethyl-3-pentanone
- CAS No.:565-80-0
- Molecular Formula:C7H14O
- Molecular Weight:114.188
- Hs Code.:2914.19 Oral rat LD50: 3536 mg/kg
- European Community (EC) Number:209-294-7
- NSC Number:14662
- UNII:7AAP3A50IG
- DSSTox Substance ID:DTXSID3038771
- Nikkaji Number:J6.514E
- Wikidata:Q27159900
- Metabolomics Workbench ID:44873
- ChEMBL ID:CHEMBL3560924
- Mol file:565-80-0.mol
Synonyms:2,4-DIMETHYL-3-PENTANONE;565-80-0;Diisopropyl ketone;2,4-Dimethylpentan-3-one;Isobutyrone;Isopropyl ketone;3-Pentanone, 2,4-dimethyl-;DIISOPROPYLKETONE;2,4-dimethyl-pentan-3-one;EINECS 209-294-7;NSC 14662;(iso-C3H7)2CO;UNII-7AAP3A50IG;PM 2763;7AAP3A50IG;DTXSID3038771;3-PENTANONE,2,4-DIMETHYL;NSC-14662;MFCD00008918;isopropylketone;diisopropylketon;di-iso-propyl ketone;3-Pentanone,4-dimethyl-;SCHEMBL25173;2, 4-Dimethyl-3-pentanone;Dimethyl-3-pentanone, 2,4-;CHEMBL3560924;DTXCID1018771;CHEBI:87754;2,4-Dimethyl-3-pentanone, 98%;NSC14662;Tox21_303876;AKOS005206866;NCGC00357269-01;CAS-565-80-0;LS-101945;D0770;FT-0610158;EN300-67377;2,4-Dimethyl-3-pentanone (diisopropyl ketone);A831104;Q27159900


 F,
F,  Xn
Xn


