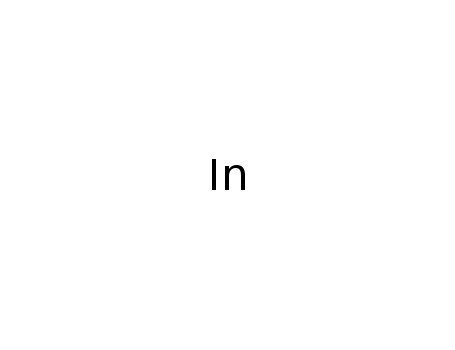10.1021/ja029276s
The research aimed to develop a highly regioselective metal-mediated allylation reaction in aqueous media for the synthesis of R-adduct homoallylic alcohols, which are important building blocks in the organic synthesis of biologically active molecules. The study proposed a new mechanism for the formation of these synthetically challenging molecules, suggesting that the reaction occurs in two stages: the initial formation of a γ-adduct followed by a rearrangement to form the thermodynamically more stable R-adduct. The research utilized indium, zinc, and tin as catalysts and involved a variety of aldehydes and allylic halides. The study concluded that the use of these metals in water, with specific conditions regarding the amount of water and temperature, could yield R-homoallylic alcohols with high selectivity and moderate to good yields, providing a new mechanistic understanding of metal-mediated allylation reactions and their high R-selectivity.