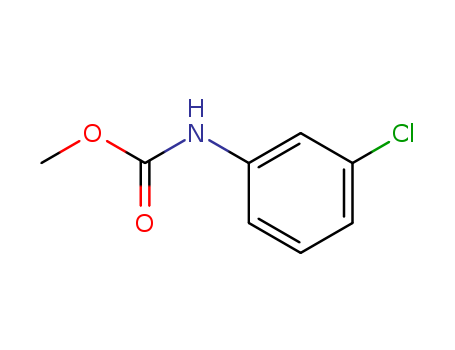
- Chemical Name:Methyl N-(3-chlorophenyl)carbamate
- CAS No.:2150-88-1
- Molecular Formula:C8H8 Cl N O2
- Molecular Weight:185.61
- Hs Code.:
- European Community (EC) Number:633-666-1
- DSSTox Substance ID:DTXSID40175842
- Nikkaji Number:J540.716H
- Wikidata:Q83046171
- Mol file:2150-88-1.mol
Synonyms:METHYL N-(3-CHLOROPHENYL)CARBAMATE;2150-88-1;Methyl (3-chlorophenyl)carbamate;Carbamic acid, 3-chlorophenyl-, methyl ester;Enamine_005868;Cambridge id 5103637;methyl 3-chlorophenylcarbamate;SCHEMBL1188370;DTXSID40175842;SEPMCAVKHUPSMA-UHFFFAOYSA-N;HMS1410K16;methyl-N-(3-chlorophenyl)carbamate;AKOS001022202;3-Chlorophenylcarbamic acid methyl ester;IDI1_008103;(3-Chloro-phenyl)-carbamic acid methyl ester;F81747;Z56754690;F3035-0947