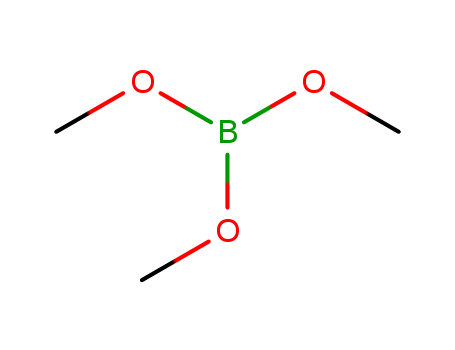
- Chemical Name:Trimethyl borate
- CAS No.:121-43-7
- Deprecated CAS:31649-91-9,63156-11-6,63156-11-6
- Molecular Formula:C3H9BO3
- Molecular Weight:103.914
- Hs Code.:2920.90
- European Community (EC) Number:204-468-9,934-685-0,693-032-5
- ICSC Number:0593
- NSC Number:777
- UN Number:2416
- UNII:82U64J6F5N
- DSSTox Substance ID:DTXSID0037738
- Nikkaji Number:J2.484H
- Wikipedia:Trimethyl_borate
- Wikidata:Q423710
- Mol file:121-43-7.mol
Synonyms:trimethyl borate


 Xn,
Xn, F
F


