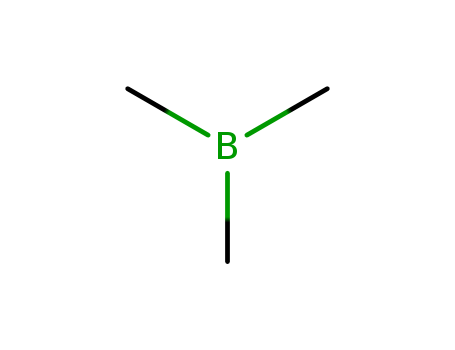
- Chemical Name:Trimethylborane
- CAS No.:593-90-8
- Molecular Formula:C3H9 B
- Molecular Weight:55.9155
- Hs Code.:
- European Community (EC) Number:209-816-3
- DSSTox Substance ID:DTXSID90208038
- Nikkaji Number:J2.700F
- Wikipedia:Trimethylborane
- Wikidata:Q7842211
- Mol file:593-90-8.mol
Synonyms:Trimethylborane;Trimethylboron;593-90-8;Borane, trimethyl-;EINECS 209-816-3;MFCD00058727;Trimethyl boron;Trimethylboron, elec. gr.;C3H9B;(CH3)3B;C3-H9-B;DTXSID90208038;AKOS025295508;Trimethylboron, electronic grade, >=98.35%;Q7842211