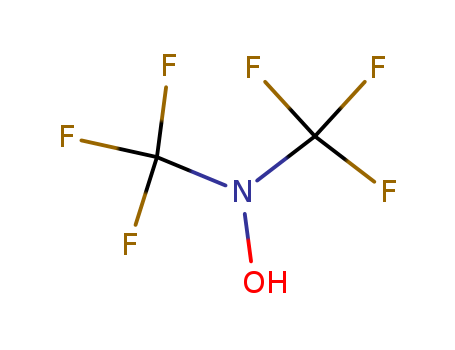
- Chemical Name:N,N-bis(trifluoromethyl)hydroxylamine
- CAS No.:359-63-7
- Molecular Formula:C2H F6 N O
- Molecular Weight:169.026
- Hs Code.:2928000090
- DSSTox Substance ID:DTXSID20371168
- Nikkaji Number:J589.535I
- Wikidata:Q81985746
- Mol file:359-63-7.mol
Synonyms:N,N-bis(trifluoromethyl)hydroxylamine;359-63-7;SCHEMBL815119;DTXSID20371168;OGYLARHBIDXOMV-UHFFFAOYSA-N;AKOS006229905;FT-0748908