10.1039/b207936d
The research focuses on the extension of the "ring switch" approach to the synthesis of glutamate antagonists, specifically utilizing δ-lactam urethanes. The study successfully employed three different types of δ-lactam urethane aldehydes (17, 26, and 59) in the synthesis process, manipulating diastereoisomeric ratios through the use of a hindered proton source to obtain homochiral products with two chiral centers. Although the δ-lactam urethane system was less versatile compared to pyroglutamate or β-lactam urethanes, the research managed to prepare a variety of glutamate antagonist homologues. The experiments involved the synthesis of compounds that mimic glutamate, the fast excitatory receptor in the brain, and are potentially useful in treating illnesses such as persistent pain, Alzheimer’s disease, epilepsy, and ischaemia. The methodology, referred to as a "ring switching" reaction, allows for the economical preparation of a large variety of homochiral compounds. The analyses used in the study included various spectroscopic techniques and chromatography to confirm the structures and purities of the synthesized compounds.
10.1039/c4ob01960a
The research focuses on the determination of the absolute configuration of phosphinic analogues of glutamate, specifically LSP1-2111, which are known to be potent agonists of metabotropic glutamate (mGlu) receptors and have shown promising in vivo activity. The study aimed to separate the mixture of two diastereomers of LSP1-2111, whose absolute and relative configurations were previously unknown, using a Crownpack CR(+) column. The absolute configuration was assessed through a diastereoselective synthesis involving various reactants such as phosphinic acid, 3-nitrobenzaldehydes, and amino acids. The experiments included radical processes, condensation, acid hydrolysis, and the use of chiral HPLC for separation. The analysis involved optical rotation, 1H NMR, 31P NMR, and X-ray crystallography to characterize the diastereomers and determine their absolute configuration. The study also investigated the biological activity of the separated L-stereomers, finding that they activated the mGlu4 receptor with varying EC50 values.
10.1021/jm8012882
The study focuses on the synthesis, biological, and physicochemical properties of novel isothiourea derivatives, specifically 3-allyl-1,1-dibenzyl-2-ethyl-isothiourea salts (1: hydrochloride, 2: hydrobromide, and 3: hydroiodide), which were developed as potential neuroprotectors and cognition enhancers. These compounds were evaluated for their ability to inhibit glutamate-stimulated calcium ion uptake in rat brain synaptosomes, interact with NMDA receptors, and their effects on AMPA receptor transmembrane currents induced by kainic acid and glutamate in Purkinje neurons. The study also included the growth of single crystals and X-ray diffraction experiments to determine the crystal structures of these salts, analysis of their solubility and partitioning properties in water and n-octanol, and assessment of their chemical stability in pH 7.4 phosphate buffer at 25 °C. The main purpose of these chemicals was to investigate their potential as therapeutic agents for neurological disorders by targeting ionotropic glutamate receptors, which play crucial roles in neuronal signaling, memory consolidation, and synaptic plasticity.
10.1021/ol800544a
The study focuses on the intramolecular Nicholas reaction, a method for the stereoselective synthesis of 5-alkynylproline derivatives, which are important structural components in natural and bioactive products. The researchers used N,N-acyl-diprotected ω-semialdehydes derived from glutamic acid as starting materials. Key chemicals involved in the process include propargylic alcohols, Co2(CO)6 (a cobalt complex used for complexation), and various N-protecting groups such as tosyl and benzoyl derivatives. These chemicals served the purpose of controlling the stereochemistry during the ring formation, allowing for the selective synthesis of specific isomers of 5-alkynylproline derivatives. The study also utilized semiempirical calculations to support the observed stereochemistry and to understand the influence of different N-protecting groups on the cyclization reaction. The goal was to develop a practical method for constructing 2,5-dialkylpyrrolidines in their enantiomeric forms, which are versatile intermediates for the synthesis of a wide variety of related molecules.
10.1016/S0957-4166(97)00121-3
The research aimed to develop a stereocontrolled synthesis method for producing stereoregular, chiral analogs of nylon 5,5 and nylon 5,6, utilizing L-glutamic acid as a chiral template. The study focused on achieving stereocontrol in the synthesis of these polymers through chemoselective condensation of the ester group with aminoalcohols, leading to the formation of N-(hydroxyalkyl)amides. These amides were further functionalized by converting the alcohol function into an amine through a series of reactions involving tosylation, azide substitution, and hydrogenolysis. The resulting amino lactones were then used in polycondensation to yield the final crystalline polyamides. The chemicals used in this process included L-glutamic acid, pentachlorophenyl ester, aminoalcohols, ethyldiisopropylamine (EDPA), tosyl chloride, sodium azide, and palladium on carbon for hydrogenolysis, among others. The conclusions of the research were that the synthesized polyamides displayed high optical rotation values, indicating their stereoregularity, and were highly crystalline as confirmed by X-ray diffraction and DSC analysis.
10.1021/jm0491952
The research aimed to develop potent and selective GluR5 kainate receptor antagonists for the treatment of pain. The study focused on two competitive GluR5 KA receptor antagonists, amino acids 5 and 7, which showed high affinity for the GluR5 receptor over other glutamate receptors. Their ester prodrugs, 6 and 8, were tested for oral activity in three animal models of pain: formalin-induced paw licking, carrageenan-induced thermal hyperalgesia, and capsaicin-induced mechanical hyperalgesia. The synthesis involved various steps such as selective removal of methyl carbamate protecting groups, reduction of ketones, Mitsunobu reactions, and tetrazole formation. The study concluded that prodrugs 6 and 8 demonstrated oral efficacy in the three animal models of pain, suggesting their potential as treatments for pain conditions.
10.1016/S0957-4166(01)00251-8
Muricatacin is a bioactive hydroxy lactone compound that is isolated from the plant Annona muricata. It is mentioned in the research as a compound with physiological activity. The study focuses on the synthesis of aza-muricatacin, a non-natural aza-analogue of muricatacin, which also exhibits interesting cytotoxic activity. The research aims to synthesize different diastereoisomers of aza-muricatacin, specifically the (5S,6S)- and (5S,6R)-isomers, starting from a common precursor derived from L-glutamic acid.
10.1016/j.tet.2007.03.116
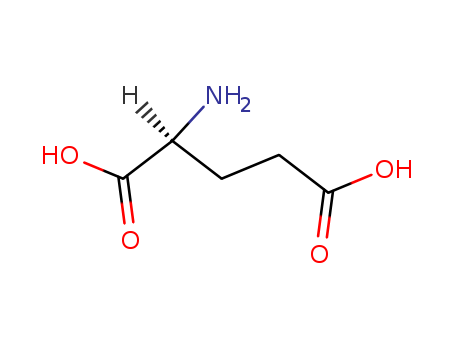
The research focuses on the novel synthesis of 2-aminopentanedinitriles from 2-(bromomethyl)aziridines and their subsequent transformation into 2-imino-5-methoxypyrrolidines and 5-methoxypyrrolidin-2-ones. The study explores an unprecedented reaction mechanism involving base-induced ring opening of intermediate 2-(cyanomethyl)aziridines into allylamines, followed by migration of the double bond towards aldimines via enamine intermediates. The synthesized aminopentanedinitriles serve as precursors for the preparation of glutamic acid derivatives, which are significant in the central nervous system as excitatory neurotransmitters. The experiments utilized reactants such as 1-arylmethyl-2-(bromomethyl)aziridines, potassium cyanide in DMSO, and sodium methoxide in methanol. The analyses included column chromatography for purification, and various spectroscopic techniques such as NMR, IR, and MS for structural characterization and confirmation of the synthesized compounds.
10.1016/0957-4166(95)00209-8
The study focuses on the asymmetric synthesis of all four isomers of 4-amino-4-carboxyproline, which are novel conformationally restricted analogues of glutamic acid. The researchers used trans-4-hydroxy-L-proline as the homochiral starting material and employed the Bucherer-Bergs reaction as the key step to form spirohydantoin rings. The study resulted in the successful synthesis of the target compounds (2S,4S)-3, (2S,4R)-4, and their corresponding enantiomers, with high enantiomeric purity (e.e. >95%). The structures and stereochemistries of these compounds were determined and confirmed using NMR studies, including NOE measurements and 1H-NMR spectra analyses.


 Xi
Xi


