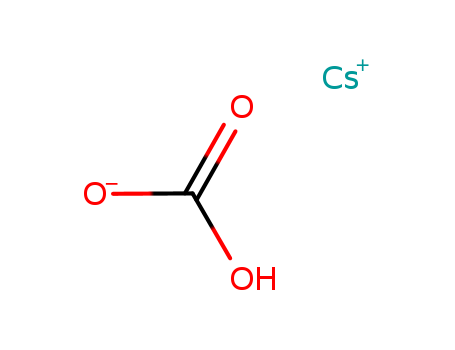
- Chemical Name:CESIUM BICARBONATE, PURE, 99.9%
- CAS No.:15519-28-5
- Molecular Formula:CH2 O3 . Cs
- Molecular Weight:193.923
- Hs Code.:28369990
- European Community (EC) Number:239-554-5,249-784-8
- DSSTox Substance ID:DTXSID70165852
- Nikkaji Number:J8.579K
- Wikipedia:Caesium carbonate,Caesium bicarbonate,Caesium_bicarbonate
- Wikidata:Q5017043
- Mol file:15519-28-5.mol
Synonyms:Carbonicacid, monocesium salt (8CI,9CI); Cesium bicarbonate; Cesium hydrogen carbonate;Cesium hydrogen carbonate (CsHCO3)