- Vanadium
- Vanadium (III) oxide
- Vanadium boride (VB)
- Vanadium boride (VB2)
- Vanadium carbides
- Vanadium dichloride
- Vanadium dihydroxide
- Vanadium fluoride
- VANADIUM GALLIDE
- Vanadium hydroxide oxide phosphate (V6(OH)3O3(PO4)7)
- 131463-67-7titanium dioxide
- 131466-61-0(3R)-3-(tert-Butyldimethylsilyloxy)glutaric acid -1-((R)-(-)-mandelic acid ester
- 1314-68-7Rhenium oxide (Re2O7)
- 131469-76-63-Cyclopropyl-2,2-dimethyl-propionic acid
- 131470-66-1Stannane,tributyl(5,6-dihydro-1,4-dioxin-2-yl)-
- 131472-28-1Benzenecarboximidamide,4-(trifluoromethyl)-
- 131477-20-8D-Proline ethyl ester hydrochloride
- 1314-80-3Phosphorus pentasulfide
- 13148-05-5Butanoic acid,3-oxo-4-(triphenylphosphoranylidene)-, ethyl ester
- 13148-22-61H-Phenalen-1-one,3-hydroxy-2-phenyl-
Hot Products
- 71769-53-4Potassium hydroxide
- 113440-58-7Calicheamicin
- 68955-54-4PRIMENE JM-T
- 62-74-8Acetic acid, 2-fluoro-,sodium salt (1:1)
- 294-40-6Cyclopentasiloxane
- 302-01-2Hydrazine
- 76688-72-7Emphos PS 21A
- 7719-12-2Phosphoroustrichloride
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin

|
Basic Information |

|
Post buying leads |

|
Suppliers |

| Name |
Vanadium pentoxide |
EINECS | 215-239-8 |
| CAS No. | 1314-62-1 | Density | 3.357 g/cm3 |
| PSA | 77.51000 | LogP | -0.54360 |
| Solubility | 1 g/125 mL in water | Melting Point |
690 °C |
| Formula | V2O5 | Boiling Point | 1750 °C |
| Molecular Weight | 181.88 | Flash Point | 1750oC |
| Transport Information | UN 2862 | Appearance | yellow to rust-brown or orange crystals or powder |
| Safety | 26-61-45-38-36/37-36 | Risk Codes | 36/38-68-63-51/53-48/23-37-20/22 |
| Molecular Structure |
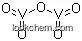
|
Hazard Symbols |
 Xi, Xi, N, N, T T
|
| Synonyms |
C.I. 77938;Divanadium pentaoxide;Pentaoxodivanadium;Vanadia;Vanadic anhydride;Vanadium oxide (V4O10);Vanadium pentoxide;Vanadium(V) oxide;Vanadium pentoxide catalyst; |
Vanadium pentoxide Consensus Reports
Vanadium pentoxide Standards and Recommendations
ACGIH TLV: TWA 0.05 mg(V2O5)/m3
NIOSH REL: (Vanadium Compound) CL 0.05 mg(V)/m3/15M
DOT Classification: 6.1; Label: Poison
Vanadium pentoxide Analytical Methods
Vanadium pentoxide Specification
The systematic name of Vanadium pentoxide is Divanadium pentaoxide. With the CAS registry number 1314-62-1, it is also named as Anhydride vanadique. The product's categories are Inorganics; Indoles; AA Standard SolutionsAnalytical Standards; Alphabetic; Reference/Calibration Standards; Standard Solutions; V; AAS; AAS CRMsSpectroscopy; AASSpectroscopy; Application CRMs; Matrix Selection; NitrateAnalytical Standards; Spectroscopy; Catalysis and Inorganic Chemistry, and the other registry numbers are 12503-98-9; 166165-37-3; 172928-47-1; 184892-22-6; 200577-85-1; 203812-34-4; 251927-12-5; 410546-90-6; 56870-07-6; 581075-33-4; 828264-64-8; 854372-97-7; 857777-38-9; 863639-26-3; 87854-55-5; 87854-56-6; 934750-46-6. Besides, it is yellow to rust-brown or orange crystals or powder, which should be stored in a cool, dry warehouse. In addition, its molecular formula is V2O5 and molecular weight is 181.88.
The other characteristics of this product can be summarized as: (1)EINECS: 215-239-8; (2)H-Bond Donor: 0; (3)H-Bond Acceptor: 5; (4)Rotatable Bond Count: 0; (5)Exact Mass: 181.862501; (6)MonoIsotopic Mass: 181.862501; (7)Topological Polar Surface Area: 77.5; (8)Heavy Atom Count: 7; (9)Complexity: 124; (10)Density: 3.357 g/cm3; (11)Melting point: 690 °C; (12)Boiling point: 1750 °C; (13)Water solubility: 1 g/125 mL.
Preparation of Vanadium pentoxide: frist, you can produce Sodium metavanadate by the reaction of Vanadium with Sodium carbonate. And then you would obtain this chemical by the reaction of Sodium metavanadate with Sulfuric acid.
.gif)
Uses of Vanadium pentoxide: this chemical can be used for decarburization, desulfuration and catalyzing in the metallurgical industry and ammonia synthesis. It is also used as coloring material of dyeing and ceramic. Additionally, it can be used as corrosion inhibitor, analytical reagent, catalyst, UV blocker, and developer. And it can be used to produce vanadium compounds.
When you are using this chemical, please be cautious about it as the following: it is harmful and toxic by inhalation and if swallowed. It is also danger of serious damage to health by prolonged exposure. In case of contact with eyes, please rinse immediately with plenty of water and seek medical advice. Moreover, it is irritating to eyes, respiratory system and skin. In case of insufficient ventilation, wear suitable respiratory equipment. And you can wear suitable protective clothing and gloves. Additionally, it is toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. It is also possible risk of harm to the unborn child and irreversible effects. Please avoid release to the environment, and refer to special instructions / safety data sheets. In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible).
People can use the following data to convert to the molecule structure.
(1)Canonical SMILES: O=[V](=O)O[V](=O)=O
(2)InChI: InChI=1S/5O.2V
(3)InChIKey: GNTDGMZSJNCJKK-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LCLo | inhalation | 500mg/m3/23M (500mg/m3) | "Vanadium Toxicology and Biological Significance," Faulkner, T.G., New York, Elsevier Science Pub. Co., 1964Vol. -, Pg. 72, 1964. | |
| guinea pig | LDLo | subcutaneous | 20mg/kg (20mg/kg) | "Vanadium Toxicology and Biological Significance," Faulkner, T.G., New York, Elsevier Science Pub. Co., 1964Vol. -, Pg. 69, 1964. | |
| human | TCLo | inhalation | 1mg/m3/8H (1mg/m3) | LUNGS, THORAX, OR RESPIRATION: COUGH LUNGS, THORAX, OR RESPIRATION: BRONCHIOLAR CONSTRICTION SENSE ORGANS AND SPECIAL SENSES: CONJUNCTIVE IRRITATION: EYE | Archives of Environmental Health. Vol. 14, Pg. 709, 1967. |
| human | TCLo | inhalation | 346mg/m3 (346mg/m3) | LUNGS, THORAX, OR RESPIRATION: COUGH LUNGS, THORAX, OR RESPIRATION: DYSPNEA LUNGS, THORAX, OR RESPIRATION: SPUTUM | AMA Archives of Industrial Health. Vol. 19, Pg. 497, 1959. |
| mouse | LD50 | intraperitoneal | 23mg/kg (23mg/kg) | Teratogenesis, Carcinogenesis, and Mutagenesis. Vol. 16, Pg. 7, 1996. | |
| mouse | LD50 | oral | 5mg/kg (5mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 26(10), Pg. 6, 1961. | |
| mouse | LD50 | subcutaneous | 10mg/kg (10mg/kg) | Zhurnal Vsesoyuznogo Khimicheskogo Obshchestva im. D.I. Mendeleeva. Journal of the D.I. Mendeleeva All-Union Chemical Society. Vol. 19, Pg. 186, 1974. | |
| rabbit | LD50 | skin | 50mg/kg (50mg/kg) | LIVER: OTHER CHANGES KIDNEY, URETER, AND BLADDER: OTHER CHANGES | National Technical Information Service. Vol. OTS0534556, |
| rat | LC50 | inhalation | 126mg/m3/6H (126mg/m3) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: DYSPNEA | National Technical Information Service. Vol. OTS0534556-1, |
| rat | LD50 | intraperitoneal | 12mg/kg (12mg/kg) | Archiv fuer Toxikologie. Vol. 16, Pg. 182, 1956. | |
| rat | LD50 | intratracheal | 6mg/kg (6mg/kg) | Bromatologia i Chemia Toksykologiczna. Vol. 11, Pg. 191, 1978. | |
| rat | LD50 | oral | 10mg/kg (10mg/kg) | BEHAVIORAL: COMA | Archiv fuer Toxikologie. Vol. 16, Pg. 182, 1956. |
| rat | LD50 | subcutaneous | 14mg/kg (14mg/kg) | BEHAVIORAL: COMA | Archiv fuer Toxikologie. Vol. 16, Pg. 182, 1956. |

