Products Categories
| CAS No.: | 54827-17-7 |
|---|---|
| Name: | Tetramethylbenzidine |
| Article Data: | 17 |
| Molecular Structure: | |
|
|
|
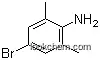
| Formula: | C16H20N2 |
| Molecular Weight: | 240.348 |
| Synonyms: | [1,1'-Biphenyl]-4,4'-diamine, 3,3',5,5'-tetramethyl-;3,3',5,5'-Tetramethyl-4,4'-biphenyldiamine;3,3',5,5'-Tetramethylbenzidine; |
| EINECS: | 259-364-6 |
| Density: | 1.071 g/cm3 |
| Melting Point: | 168-171 °C(lit.) |
| Boiling Point: | 368.623 °C at 760 mmHg |
| Flash Point: | 210.8 °C |
| Solubility: | <0.1 g/100 mL at 20 °C in water |
| Appearance: | White or light yellow solid |
| Hazard Symbols: |
 Xn, Xn,  Xi Xi
|
| Risk Codes: | 36/38-36/37/38-22 |
| Safety: | 26-36-36/37 |
| Transport Information: | UN 2796 8/PG 2 |
| PSA: | 52.04000 |
| LogP: | 4.91400 |
- 81281-59-67-Benzylideneaminotheophylline
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE
- 68439-39-4Poly(oxy-1,2-ethanediyl), alpha-(2-ethylhexyl)-omega-hydroxy-,
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 717878-06-31-(4-fluorophenyl)-4-nitro-1H-imidazole
- 914458-26-7[5-(2-fluorophenyl)-1-pentyl-1H-pyrrol-3-yl]-1-naphthalenyl-Methanone
- 548773-13-32-amino-4,6-dimethylpyrimidine-5-carboxylic acid
- 29418-31-3
1,2-Bis(2,4-dimethylphenyl)diazene

- 54827-17-7
3,3',5,5'-tetramethylbenzidine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; zinc In water; ethyl acetate at 20℃; Benzidine Rearrangement; | 96% |
| With ammonium chloride; zinc In methanol; water at 20℃; for 6h; Reflux; | 85 g |
- 24596-18-7
4-chloro-2,6-dimethylaniline

- 54827-17-7
3,3',5,5'-tetramethylbenzidine

| Conditions | Yield |
|---|---|
| With carbon dioxide; aluminium; 1-butyl-3-methylimidazolium trifluoromethanesulfonimide at 45℃; under 116262 Torr; for 10h; Ullmann reaction; | 93% |
| With 4-(3'-butyl-1'-imidazolio)-1-butanesulfonic acid hydrogen sulfate; aluminium In carbon dioxide at 45℃; under 116262 Torr; for 10h; Ullmann reaction; Supercritical conditions; | 91% |

- 54827-17-7
3,3',5,5'-tetramethylbenzidine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol at 50℃; for 1h; Inert atmosphere; | 72.4% |
- 80387-68-4
2-methylindole-3-carboxaldehyde 2,6-dimethylphenylhydrazone

A
- 54827-17-7
3,3',5,5'-tetramethylbenzidine

B
- 80387-72-0
3-(4-amino-3,5-dimethylphenyl)-2-methylindole

C
- 87-62-7
2,6-dimethylaniline

| Conditions | Yield |
|---|---|
| With PPA; Polyphosphoric acid (PPA) at 100℃; for 0.5h; | A 1.85 g B 2.53 g C 0.77 g |

- 603-77-0
(2,6-dimethylphenyl)hydrazine

- 54827-17-7
3,3',5,5'-tetramethylbenzidine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 51 percent / traces of AcOH / 0.75 h / 140 - 150 °C 2: 1.85 g / polyphosphoric acid (PPA) / 0.5 h / 100 °C View Scheme |
- 24596-19-8
4-bromo-2,6-dimethylphenylamine

A
- 54827-17-7
3,3',5,5'-tetramethylbenzidine

B
- 87-62-7
2,6-dimethylaniline

| Conditions | Yield |
|---|---|
| With sodium formate; cetyltrimethylammonim bromide; palladium on charcoal In water | A 7.7 parts (63.3%) B n/a |

- 54827-17-7
3,3',5,5'-tetramethylbenzidine

| Conditions | Yield |
|---|---|
| With uric Acid In aq. acetate buffer at 30℃; for 0.416667h; pH=4.4; |
- 87-62-7
2,6-dimethylaniline

- 54827-17-7
3,3',5,5'-tetramethylbenzidine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-Bromosuccinimide / N,N-dimethyl-formamide / 2 h / 0 - 20 °C 2: (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; caesium carbonate / 1,4-dioxane / 16 h / 75 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: N-Bromosuccinimide / N,N-dimethyl-formamide / 2 h / 0 - 20 °C 2: potassium acetate; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride / water; 1,4-dioxane / 15 h / 80 °C / Inert atmosphere 3: (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; caesium carbonate / 1,4-dioxane / 16 h / 75 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 5 steps 1: sodium tungstate (VI) dihydrate; dihydrogen peroxide / diethyl ether; water / 9 h / 20 - 30 °C 2: sodium thiosulfate; sodium hydrogen sulfide / methanol / 2.5 h / 20 - 75 °C 3: sulfuric acid / chlorobenzene / 3 h / 20 - 40 °C 4: hydrazine hydrate; hydrogenchloride; pyrographite / water / 2.5 h / 100 °C 5: sodium hydroxide / methanol / 1 h / 50 °C / Inert atmosphere View Scheme |
- 24596-19-8
4-bromo-2,6-dimethylphenylamine

- 54827-17-7
3,3',5,5'-tetramethylbenzidine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium acetate; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride / water; 1,4-dioxane / 15 h / 80 °C / Inert atmosphere 2: (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; caesium carbonate / 1,4-dioxane / 16 h / 75 °C / Inert atmosphere View Scheme |
- 1004761-68-5
2,6-dimethyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline

- 24596-19-8
4-bromo-2,6-dimethylphenylamine

- 54827-17-7
3,3',5,5'-tetramethylbenzidine

| Conditions | Yield |
|---|---|
| With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; caesium carbonate In 1,4-dioxane at 75℃; for 16h; Inert atmosphere; | 210 g |
- 108-48-5Pyridine, 2,6-dimethyl-
- 118864-75-8(1S)-1-Phenyl-1,2,3,4-tetrahydroisoquinoline
- 67375-30-8alpha-Cypermethrin
- 1317-36-8Lead monoxide
- 626-05-12,6-Dibromopyridine
- 147118-35-2Hexanoic acid,3-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-5-oxo-6-(triphenylphosphoranylidene)-,methyl ester, (3R)-
- 23680-84-44-Quinazolinamine,2-chloro-6,7-dimethoxy-
- 140-10-3trans-Cinnamic acid
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin

What can I do for you?
Get Best Price
Consensus Reports
EPA Genetic Toxicology Program. Reported in EPA TSCA Inventory.
Specification
The 3,3',5,5'-Tetramethylbenzidine, with its cas register number 54827-17-7, is a kind of white or light yellow solid. It also can be called Tetramethylbenzidine; 3,3',5,5'-Tetramethyl-(1,1'-biphenyl)-4,4'-diamine; TMB Quick; and (1,1'-Biphenyl)-4,4'-diamine, 3,3',5,5'-tetramethyl-. Tetramethylbenzidine should be stored in shady and cool warehouse and mainly used as raw material of fine chemicals and pharmaceutical intermediate.
Physical properties about 3,3',5,5'-Tetramethylbenzidine are: (1)ACD/LogP: 2.673; (2)ACD/LogD (pH 5.5): 2.63; (3)ACD/LogD (pH 7.4): 2.67; (4)ACD/BCF (pH 5.5): 57.63; (5)ACD/BCF (pH 7.4): 63.22; (6)ACD/KOC (pH 5.5): 617.03; (7)ACD/KOC (pH 7.4): 676.88; (8)#H bond acceptors: 2; (9)#H bond donors: 4; (10)#Freely Rotating Bonds: 3; (11)Index of Refraction: 1.618; (12)Molar Refractivity: 78.617 cm3; (13)Molar Volume: 224.379 cm3; (14)Polarizability: 31.166 10-24cm3; (15)Surface Tension: 45.5379981994629 dyne/cm; (16)Density: 1.071 g/cm3; (17)Flash Point: 210.807 °C; (18)Enthalpy of Vaporization: 61.53 kJ/mol; (19)Boiling Point: 368.623 °C at 760 mmHg; (20)Vapour Pressure: 0 mmHg at 25°C
When you are using this chemical, please be cautious about it as the following:
1. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice;
2. Wear suitable protective clothing;
3. Wear suitable protective clothing and gloves;
You can still convert the following datas into molecular structure:
(1)InChI=1S/C16H20N2/c1-9-5-13(6-10(2)15(9)17)14-7-11(3)16(18)12(4)8-14/h5-8H,17-18H2,1-4H3;
(2)InChIKey=UAIUNKRWKOVEES-UHFFFAOYSA-N;
(3)Smilesc1(c(cc(c2cc(c(N)c(c2)C)C)cc1C)C)N;
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 135mg/kg (135mg/kg) | Progress in Mutation Research. Vol. 1, Pg. 682, 1981. | |
| quail | LD50 | oral | > 316mg/kg (316mg/kg) | Ecotoxicology and Environmental Safety. Vol. 6, Pg. 149, 1982. |