Products Categories
| CAS No.: | 755037-03-7 |
|---|---|
| Name: | Regorafenib |
| Article Data: | 30 |
| Molecular Structure: | |
|
|
|
| Formula: | C21H15ClF4N4O3 |
| Molecular Weight: | 482.822 |
| Synonyms: | 4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]-3-fluorophenoxy]-N-methylpyridine-2-carboxamide;4-(4-(3-(4-Chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpicolinamide; |
| EINECS: | 815-051-1 |
| Density: | 1.491 g/cm3 |
| Melting Point: | 206.0 to 210.0 °C |
| Boiling Point: | 513.4 °C at 760 mmHg |
| Flash Point: | 264.3 °C |
| Solubility: | DMSO |
| PSA: | 92.35000 |
| LogP: | 6.22570 |
- 81281-59-67-Benzylideneaminotheophylline
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 914458-26-7[5-(2-fluorophenyl)-1-pentyl-1H-pyrrol-3-yl]-1-naphthalenyl-Methanone
- 894852-01-87-BROMO-2,2-DIMETHYL-2H-PYRIDO[3,2-B][1,4]OXAZIN-3(4H)-ONE
- 90221-55-92-bromo-5-methylbenzaldehyde
- 885590-99-82,3-DIFLUORO-4-IODOBENZALDEHYDE
- 97730-31-9(S)-4'-(2-Methylbutyl)Biphenyl-4-Carbonitrile
- 757251-39-1
4-(4-amino-3-fluorophenoxy)pyridine-2-carboxylic acid methyl amide

- 42558-54-3
Methyl 4-methyl-3-oxopentanoate

- 320-51-4
4-chloro-3-trifluoromethyl-aniline

- 755037-03-7
regorafenib

| Conditions | Yield |
|---|---|
| With dmap In N,N-dimethyl-formamide at 140℃; for 4h; Temperature; Green chemistry; | 96.8% |

- 757251-39-1
4-(4-amino-3-fluorophenoxy)pyridine-2-carboxylic acid methyl amide

- 868-84-8
S,S-dimethyl dithiocarbonate

- 320-51-4
4-chloro-3-trifluoromethyl-aniline

- 755037-03-7
regorafenib

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydroxide In tetrahydrofuran at 35℃; for 1.33333h; Green chemistry; | 96.2% |

- 757251-39-1
4-(4-amino-3-fluorophenoxy)pyridine-2-carboxylic acid methyl amide

- 327-78-6
4-chloro-3-(trifluoromethyl)phenyl isocyanate

- 755037-03-7
regorafenib

| Conditions | Yield |
|---|---|
| In ethyl acetate at 20℃; for 1.5h; | 94.5% |
| In dichloromethane at 0 - 20℃; for 16h; Inert atmosphere; | 90% |
| In dichloromethane for 24h; | 87% |
- 399-95-1
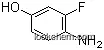
4-amino-3-fluorophenol

- 755037-03-7
regorafenib

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: Reflux 2.1: 1-methyl-pyrrolidin-2-one / 100 °C 2.2: 4.17 h / 100 °C 2.3: 0.17 h / 80 °C 3.1: toluene; tetrahydrofuran / 4.5 h / 20 °C 3.2: 2.25 h View Scheme | |
| Multi-step reaction with 3 steps 1.1: cyclohexane / Reflux 2.1: 1-methyl-pyrrolidin-2-one / 100 °C 2.2: 3.67 h / 100 °C 2.3: 0.17 h / 80 °C 3.1: toluene; tetrahydrofuran / 4.5 h / 20 °C 3.2: 2.25 h View Scheme | |
| Multi-step reaction with 3 steps 1.1: Reflux 2.1: 1-methyl-pyrrolidin-2-one / 100 °C 2.2: 5.5 h / 100 °C 2.3: 0.17 h / 80 °C 3.1: toluene; tetrahydrofuran / 4.5 h / 20 °C 3.2: 2.25 h View Scheme | |
| Multi-step reaction with 2 steps 1.1: potassium tert-butylate / N,N-dimethyl acetamide / 0.42 h / 0 °C 1.2: 16 h / 100 °C 2.1: toluene / 72 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: dichloromethane / 6 h / Reflux 2: potassium tert-butylate; potassium carbonate / N,N-dimethyl-formamide / 3 h / 90 °C View Scheme |
- 1338722-52-3
C12H16FNO

- 755037-03-7
regorafenib

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 1-methyl-pyrrolidin-2-one / 100 °C 1.2: 4.17 h / 100 °C 1.3: 0.17 h / 80 °C 2.1: toluene; tetrahydrofuran / 4.5 h / 20 °C 2.2: 2.25 h View Scheme |
- 1338722-53-4
C11H14FNO

- 755037-03-7
regorafenib

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 1-methyl-pyrrolidin-2-one / 100 °C 1.2: 5.5 h / 100 °C 1.3: 0.17 h / 80 °C 2.1: toluene; tetrahydrofuran / 4.5 h / 20 °C 2.2: 2.25 h View Scheme |
- 394-41-2
3-fluoro-4-nitrophenol

- 755037-03-7
regorafenib

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: hydrogen / palladium 10% on activated carbon / ethyl acetate / 4 h 2.1: potassium tert-butylate / N,N-dimethyl acetamide / 0.42 h / 0 °C 2.2: 16 h / 100 °C 3.1: toluene / 72 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: potassium carbonate / diethylene glycol dimethyl ether / 6 h / Reflux 2: hydrogen / methanol / 3 h / 20 °C / 1520.1 Torr / Autoclave 3: tetrahydrofuran / 5 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: potassium carbonate / N,N-dimethyl-formamide / 3 h / 110 °C / Inert atmosphere 2: hydrogen; 5%-palladium/activated carbon / ethyl acetate / 1125.11 Torr 3: dichloromethane / 3 h / 0 - 25 °C View Scheme |
- 98-98-6
2-Picolinic acid

- 755037-03-7
regorafenib

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: sodium bromide / chlorobenzene / 0.25 h / 50 °C 1.2: 23 h / 85 °C 2.1: water; toluene / 7 h / 20 °C 3.1: potassium tert-butylate / N,N-dimethyl-formamide / 0.5 h / 20 °C 3.2: 5 h / 100 °C 4.1: sulfuric acid; nitric acid / water / 1.5 h / -10 - 0 °C 5.1: ammonium chloride; iron; hydrogenchloride / water; ethanol / 0.25 h / Reflux 6.1: ethyl acetate / 1.5 h / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: thionyl chloride / N,N-dimethyl-formamide 2.1: sodium hydroxide / tetrahydrofuran / 3 h / -5 °C 3.1: potassium tert-butylate / dimethyl amine / 2 h / 20 °C / Inert atmosphere 3.2: 85 °C / Inert atmosphere 4.1: dichloromethane / 16 h / 0 - 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1.1: thionyl chloride; sodium bromide / N,N-dimethyl-formamide / 30 h / 80 °C 2.1: sodium hydroxide / tetrahydrofuran / 3 h / -5 °C 3.1: potassium tert-butylate / dimethyl amine / 2 h / 20 °C / Inert atmosphere 3.2: 85 °C / Inert atmosphere 4.1: dichloromethane / 16 h / 0 - 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 5 steps 1.1: N,N-dimethyl-formamide; thionyl chloride / 18 h / 70 °C / Inert atmosphere; Schlenk technique 2.1: tetrahydrofuran; methanol; water / 1 h / 0 - 10 °C / Inert atmosphere; Schlenk technique 3.1: potassium tert-butylate; potassium carbonate / N,N-dimethyl-formamide / 0.5 h / 0 °C / Inert atmosphere; Schlenk technique 3.2: 16 h / 110 °C / Inert atmosphere; Schlenk technique 4.1: sulfuric acid; nitric acid / water / 1 h / 0 °C / Inert atmosphere; Schlenk technique 4.2: 1 h / 80 °C / Inert atmosphere; Schlenk technique 5.1: dichloromethane; ethyl acetate / 18 h / 20 °C / Inert atmosphere; Schlenk technique View Scheme |

- 755037-03-7
regorafenib

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: water; toluene / 7 h / 20 °C 2.1: potassium tert-butylate / N,N-dimethyl-formamide / 0.5 h / 20 °C 2.2: 5 h / 100 °C 3.1: sulfuric acid; nitric acid / water / 1.5 h / -10 - 0 °C 4.1: ammonium chloride; iron; hydrogenchloride / water; ethanol / 0.25 h / Reflux 5.1: ethyl acetate / 1.5 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: tetrahydrofuran / 16.42 h / 0 - 20 °C / Inert atmosphere 2.1: potassium tert-butylate / N,N-dimethyl-formamide / 3.03 h / Inert atmosphere 2.2: 10 h / 90 °C / Inert atmosphere 3.1: dichloromethane / 24 h View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium hydroxide / tetrahydrofuran / 3 h / -5 °C 2.1: potassium tert-butylate / dimethyl amine / 2 h / 20 °C / Inert atmosphere 2.2: 85 °C / Inert atmosphere 3.1: dichloromethane / 16 h / 0 - 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1.1: tetrahydrofuran; methanol; water / 1 h / 0 - 10 °C / Inert atmosphere; Schlenk technique 2.1: potassium tert-butylate; potassium carbonate / N,N-dimethyl-formamide / 0.5 h / 0 °C / Inert atmosphere; Schlenk technique 2.2: 16 h / 110 °C / Inert atmosphere; Schlenk technique 3.1: sulfuric acid; nitric acid / water / 1 h / 0 °C / Inert atmosphere; Schlenk technique 3.2: 1 h / 80 °C / Inert atmosphere; Schlenk technique 4.1: dichloromethane; ethyl acetate / 18 h / 20 °C / Inert atmosphere; Schlenk technique View Scheme |
- 220000-87-3
4-chloro-N-methylpicolinamide

- 755037-03-7
regorafenib

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: potassium tert-butylate / N,N-dimethyl-formamide / 0.5 h / 20 °C 1.2: 5 h / 100 °C 2.1: sulfuric acid; nitric acid / water / 1.5 h / -10 - 0 °C 3.1: ammonium chloride; iron; hydrogenchloride / water; ethanol / 0.25 h / Reflux 4.1: ethyl acetate / 1.5 h / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: potassium hydroxide / N,N-dimethyl-formamide / 0.5 h / 0 - 50 °C 1.2: 100 °C 2.1: sulfuric acid; nitric acid / 1.5 h / -10 - 0 °C 3.1: iron; ammonium chloride; hydrogenchloride / water; ethanol / 1 h / Reflux 4.1: ethyl acetate / 0.5 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: potassium tert-butylate / N,N-dimethyl-formamide / 3.03 h / Inert atmosphere 1.2: 10 h / 90 °C / Inert atmosphere 2.1: dichloromethane / 24 h View Scheme |
- 115-19-53-Butyn-2-ol, 2-methyl-
- 136790-76-6Lubiprostone
- 1306-23-6Cadmium sulfide
- 57-62-5Chlorotetracycline
- 82-38-2Disperse Red 9
- 2215-89-64,4'-Oxybisbenzoic acid
- 74-11-3Benzoic acid, 4-chloro-
- 78-90-01,2-Propanediamine
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
Specification
The 4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-N-methylpyridine-2-carboxamide with CAS registry number of 755037-03-7 is also called Regorafenib. Its systematic name is called 4-(4-(((4-chloro-3-(trifluoromethyl)phenyl)carbamoyl}amino)-3-fluorophenoxy)-N-methylpyridine-2-carboxamide.
Physical properties about this chemical are: (1) ACD/LogP: 4.49; (2) # of Rule of 5 Violations: 0; (3) ACD/LogD (pH 5.5): 4.48; (4) ACD/LogD (pH 7.4): 4.48; (5) ACD/BCF (pH 5.5): 1507.8; (6) ACD/BCF (pH 7.4): 1506.05; (7) ACD/KOC (pH 5.5): 6556.04; (8) ACD/KOC (pH 7.4): 6548.43; (9) #H bond acceptors: 7; (10) #H bond donors: 3; (11) #Freely Rotating Bonds: 5; (12) Polar Surface Area: 65.98 Å2; (13) Index of Refraction: 1.615; (14) Molar Refractivity: 113.09 cm3; (15) Molar Volume: 323.6 cm3; (16) Polarizability: 44.83×10-24 cm3; (17) Surface Tension: 50.7 dyne/cm ; (18) Density: 1.491 g/cm3; (19) Flash Point: 264.3 °C ; (20) Enthalpy of Vaporization: 78.48 kJ/mol ; (21) Boiling Point: 513.4 °C at 760 mmHg; (22) Vapour Pressure: 1.19E-10 mmHg at 25°C.
You can still convert the following datas into molecular structure:
(1) InChI: InChI=1S/C21H15ClF4N4O3/c1-27-19(31)18-10-13(6-7-28-18)33-12-3-5-17(16(23)9-12)30-20(32)29-11-2-4-15(22)14(8-11)21(24,25)26/h2-10H,1H3,(H,27,31)(H2,29,30,32).
-
Premium Related Products