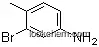
7745-91-7Relevant articles and documents
Preparation method of 2 -bromo -4-chlorobenzaldehyde
-
, (2021/08/25)
The invention relates to a preparation method of 2 -bromo -4-chlorobenzaldehyde and belongs to the field of medicinal chemistry. The preparation method can obtain 2 -bromo -4-chlorobenzaldehyde by taking nitrotoluene as a starting material through substitution, reduction, substitution, substitution, substitution, hydrolysis and elimination reaction. Compared with the prior art, the method shortens the reaction time, the reaction temperature is higher 120 °C or higher, the reaction conditions are mild, the yield of the obtained product is about 70% and more than 90%. The reaction temperature is reduced, high-boiling-point solvent is not needed to participate in the reaction, the post-treatment is simple, and the method is more suitable for industrial production with strict safety and environmental protection.
Iridium-catalyzed transfer hydrogenation of nitroarenes to anilines
Chen, Shujie,Lu, Guoping,Cai, Chun
, p. 5360 - 5365 (2015/07/07)
A simple and general homogeneous catalyst system composed of commercially available [Ir(cod)Cl]2 and 1,10-phenanthroline has been developed for the selective transfer hydrogenation of nitroarenes to anilines. It utilized the readily accessible 2-propanol as a hydrogen donor and had wide substrate scope. A careful mechanistic investigation through real-time detection and a series of controlled experiments with possible intermediates was also carried out, which showed that the transformation proceeds via both phenylhydroxylamine and azobenzene intermediates and the reduction of hydrazobenzene leading to aniline might be the rate-determining step.
Structure elucidation and enantioselective total synthesis of the HMG-CoA reductase inhibitors FR901512 and FR901516
Inoue, Masahiro,Nakada, Masahisa
scheme or table, p. 3694 - 3707 (2010/03/30)
The enantioselective total synthesis of the potent HMGCoA reductase inhibitors FR901512 (1) and FR901516 (2) is reviewed. FR901512 was prepared in 15 steps from commercially available compound via 2 in 16.3% overall yield (89% average yield). This study validated the applicability and reliability of the catalytic asymmetric Nozaki-Hiyama reactions that were developed by us. These reactions enabled the concise, efficient, and protecting-group-free enantioselective total syntheses of these new statins.