- Schöllkopf Bis-Lactim Amino Acid Synthesis
-
Schöllkopf Bis-Lactim Amino Acid Synthesis
U. Schöllkopf et al., Angew. Chem. Int. Ed. 18, 863 (1979); 20, 798 (1981).
Asymmetric amino acid synthesis via diastereoselective alkylation of the lithiated bis-lactim ether (derived from L-Val and Gly or Ala) by an electrophile. Subsequent acid hydrolysis liberatesL-Val-OCH3 and the (R)-α-substituted amino acid ester. When the bis-lactim is generated from D-Val, the (S)-enantiomer forms:
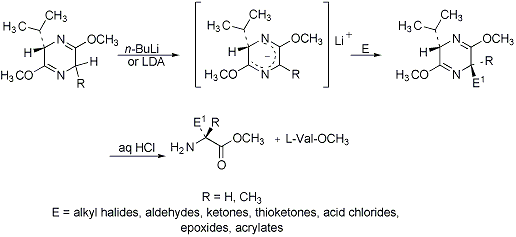
Synthetic applications: S. Kotha, A. Kuki, Chem. Commun. 1992, 404; M. S. Allen et al., Synth. Commun. 22, 2077 (1992). Isotopic labeling: N. R. Thomas, D. Gani, Tetrahedron 47, 497 (1991). Reviews: U. Schöllkopf, Top. Curr. Chem. 109, 65-84 (1983); idem, Pure Appl. Chem. 55, 1799-1806 (1983); R. M. Williams, Synthesis of Optically Active α-Amino Acids (Pergamon, New York, 1989) pp 1-33.
Prev: Semmler-Wolff Reaction
Next: Scholl Reaction - 【Back】【Close 】【Print】【Add to favorite 】
- Related information
- Market Quotations of Adipic Acid on January 12th
- Market Quotations of Adipic Acid on January 11th
- International Priceso of Synthesis Ammonia in the Falling Trends
- Market Quotations of Adipic Acid on January 10th
- Market Quotations of Adipic Acid on January 9th
- Market Quotations of Adipic Acid on January 4th
- Prices of Sulfuric Acid Continues Its Falling Trends
- Market Quotations of Adipic Acid on December 31th
- Market Quotations of Adipic Acid on December 30th
- Market Quotations of Adipic Acid on December 28th
-
Health and Chemical more >
-
Hot Products
- 39270-45-6 2-(4-METHYL-1,4-DIAZEPAN-1-YL)ETHYLAMINE
- 75-58-1 Tetramethylammonium iodide
- 122-01-0 Benzoylchloride, 4-chloro-
- 4252-78-2 2,2',4'-Trichloroacetophenone
- 32863-01-7 4-(2-HYDROXY-ETHYL)-CYCLOHEXANONE
- 168547-43-1 AMMONIUM NIOBATE(V) OXALATE HYDRATE 99&
- 1830-54-2 Dimethyl 1,3-acetonedicarboxylate
- 43143-11-9 [2,2'-dithiobis[pyridine] 1,1'-dioxide-O,O',S][sulphato(2-)-O]magnesium
- 25013-16-5 Phenol,(1,1-dimethylethyl)-4-methoxy-
- 67165-56-4 Diclofensine
- 497181-27-8 3,5-Dibromo-2-fluorobenzyl alcohol
- 175837-01-1 1-Acetyl-5-(2-aminopropyl)-2,3-dihydro-1H-indole-7-carbonitrile


