
Tetrahedron p. 3471 - 3478 (1998)
Update date:2022-08-11
Topics:
 Kardassis, Georgios
Kardassis, Georgios
 Brungs, Peter
Brungs, Peter
 Steckhan, Eberhard
Steckhan, Eberhard
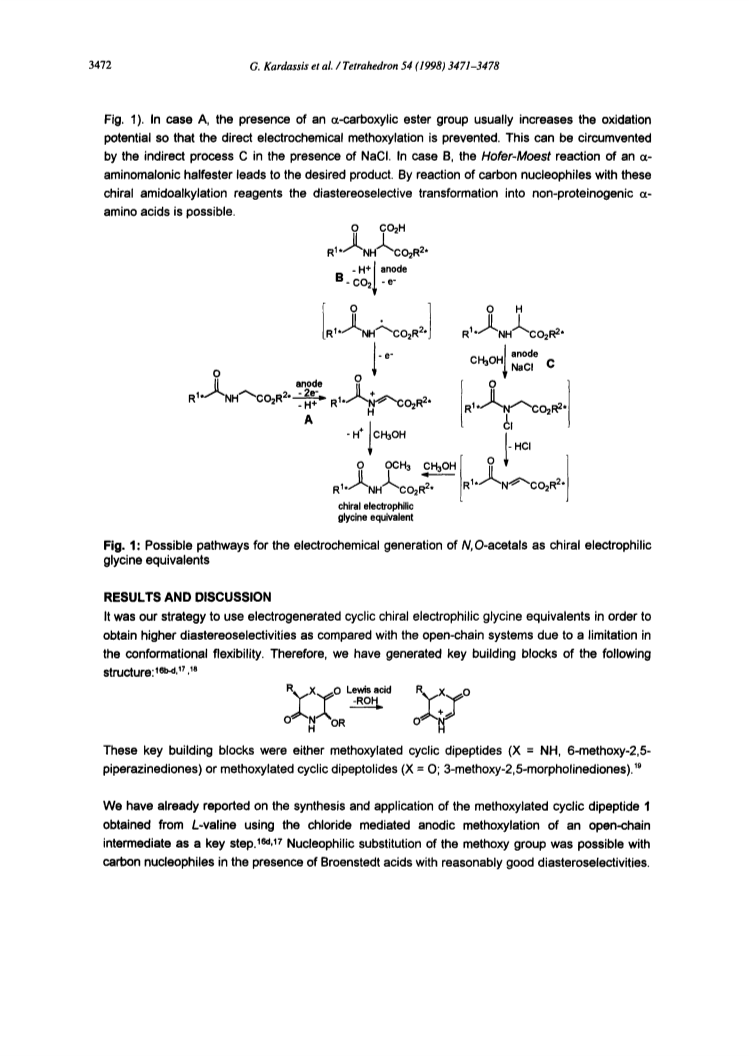
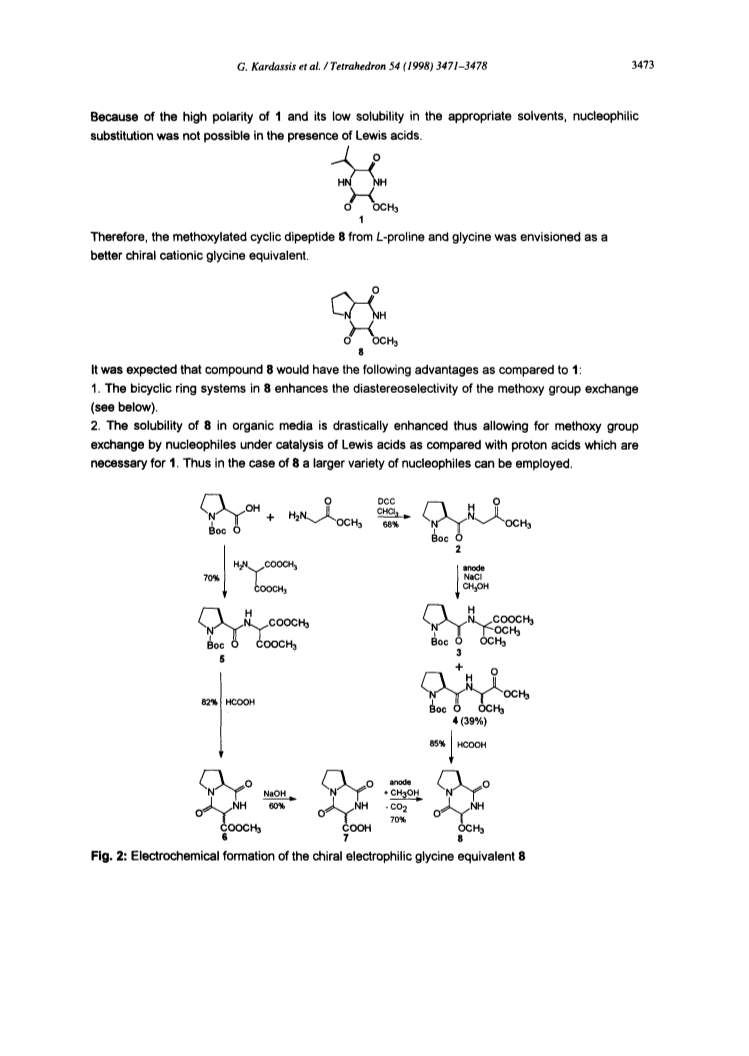
The cyclic N,O-acetal cyclo(L-Pro-Gly(OMe)OMe (8) has proved to be an effective chiral electrophilic glycine equivalent which is applicable in nucleophilic substitution reactions not only under Broenstedt acid catalysis but also under Lewis acid catalysis with excellent diastereoselectivities. This chiral building block can easily be obtained by electrochemical methoxylative decarboxylation of the cyclic dipeptide 6 generated from L-proline and aminomalonic diester. Thus, enantiomerically pure D-allyl glycine has been generated.
View More





Wuxi Forest Biological Co.,Ltd
Contact:+86-510-81602300
Address:Room 317,Building D, No.159 middle Chengjiang Road,Jiangyin Wuxi city.
Shanghai Sharing technologies Co., Ltd.
Contact:86-021-66787223
Address:No11, Lane 225, Jinxiang Road,Pudong district
Jiaxing Trustworthy Import And Export Co.,Ltd
Contact:+86-573-82030555
Address:Room 1202, Unit B, Charming plaza,No.1558 East Zhongshan Road , Jiaxing City, Zhejiang Province, China.
Shanghai Yudiao Chemistry Technology Co.,Ltd
Contact:0086-18964703211
Address:Building NO.5, NO.218,Rongtian Road,ganxiang town,Jinshan District,shanghai,201518,china
Hangzhou Yanshan Chemical Co.,Ltd.
Contact:86-571- 87698076
Address:Room 1001, #1 Building, Zhongtian MCC, No.2 Youzhinong, Wenyi West Road, Xihu District, Hangzhou, China
Doi:10.1021/acs.jmedchem.8b01746
(2019)Doi:10.1016/S0040-4039(00)89717-8
(1968)Doi:10.1080/00397911.2021.1904991
(2021)Doi:10.1016/j.bioorg.2018.09.011
(2018)Doi:10.1016/j.jorganchem.2006.10.011
(2007)Doi:10.1002/jhet.2880
(2017)