
Inorganic Chemistry Communications p. 212 - 218 (2019)
Update date:2022-08-17
Topics:
 Yanase, Ikuo
Yanase, Ikuo
 Takano, Takuya
Takano, Takuya
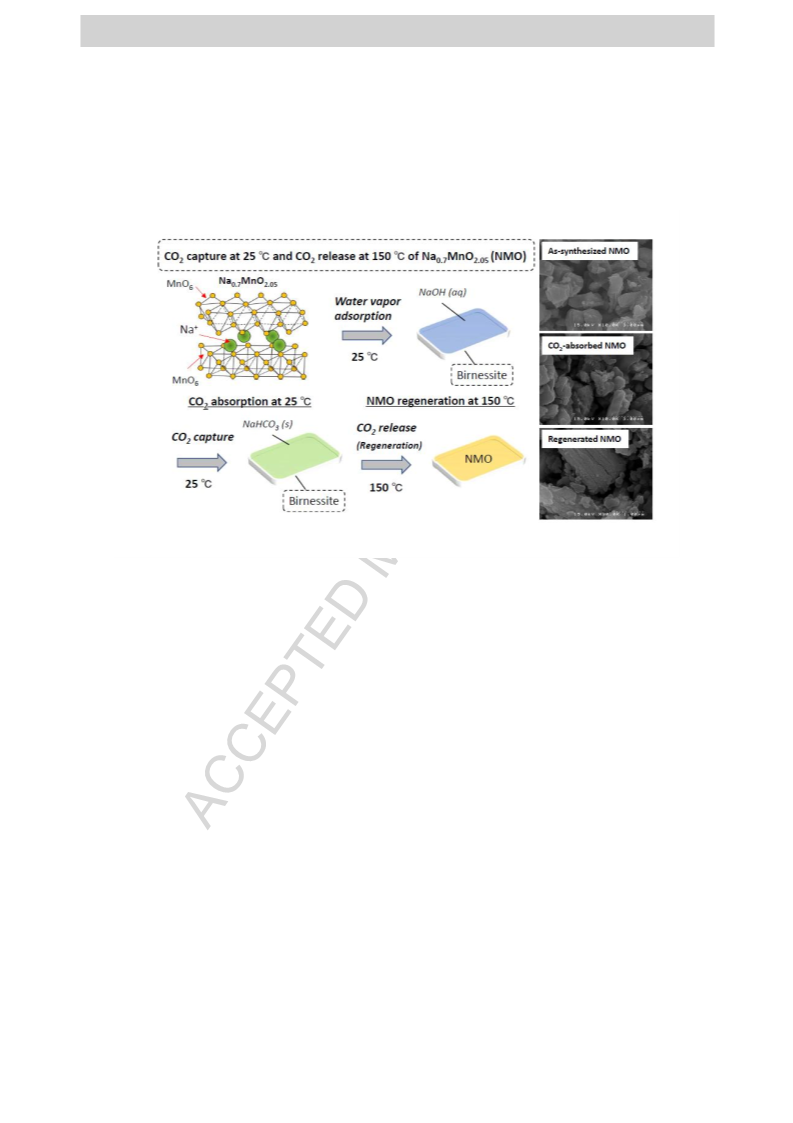
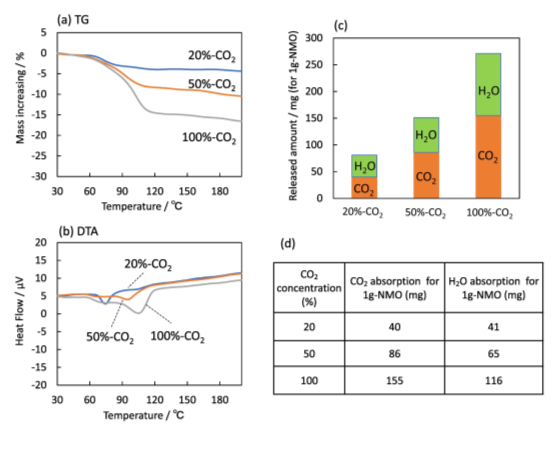
Sodium manganates with a layered structure, Na0.7MnO2.05, have been applied to a novel material for CCUS (CO2 capture, utilization, and storage), capable of capturing CO2 at 25 °C in the presence of water vapor and releasing CO2 at 150 °C. The temperatures of capturing and releasing CO2 of Na0.7MnO2.05 were remarkably lower than those of other traditional metal oxides. The CO2 absorption and desorption properties of Na0.7MnO2.05 were investigated by various methods, such as thermogravimetry, Fourier transform infrared spectroscopy, X-ray diffractometry, and gas chromatography. These investigations confirmed that Na0.7MnO2.05 absorbed CO2 at 25 °C in the presence of water vapor to produce NaHCO3 and a birnessite and the CO2 absorption was promoted by increasing relative humidity and CO2 concentration. The CO2 absorption at 25 °C of Na0.7MnO2.05 was promoted by the formation of a strong basic solution on Na0.7MnO2.05, caused by the elution of Na ions from the interlayer of Na0.7MnO2.05 into water, adsorbed on the Na0.7MnO2.05 surface. Furthermore, Na0.7MnO2.05 was regenerated by heating the CO2-absorbed Na0.7MnO2.05 at temperatures as low as 150 °C. The low-temperature regeneration indicates that Na0.7MnO2.05 can be a low-energy consumption material for capturing and releasing CO2 at low temperatures.
View More



























Shandong Hongxiang Zinc Co., Ltd
Contact:086-0311-66187879
Address:DaWang developing zone
Jiangsu Cale New Material Co.ltd
Contact:+86-515-88334667/88203550
Address:Zhongshan 3rd Road, Coastal Chemical Industry Park, Yancheng, Jiangsu, China
Contact:+86-158-05817090
Address:ROOM 9F, FLAT 2, GUODU DEVELOPING BLDG, No.182, ZHAOHUI ROAD
Contact:+86-25-52346955
Address:199,JIANYE ROAD,NANJING,CHINA
Contact:+86-021-58123769
Address:No.780 of Cailun Road,Zhangjiang Hi-tech Park,Pudong,Shanghai
Doi:10.1021/ol051129m
(2005)Doi:10.1016/S1872-2067(16)62503-2
(2016)Doi:10.1016/j.apcata.2014.07.009
(2014)Doi:10.1016/S0040-4039(90)80004-6
(1990)Doi:10.1016/0039-6028(88)90620-6
(1988)Doi:10.1016/S0040-4039(01)99714-X
(1966)