
Journal of Organic Chemistry p. 2436 - 2442 (1995)
Update date:2022-08-18
Topics:
 Maltese, M.
Maltese, M.
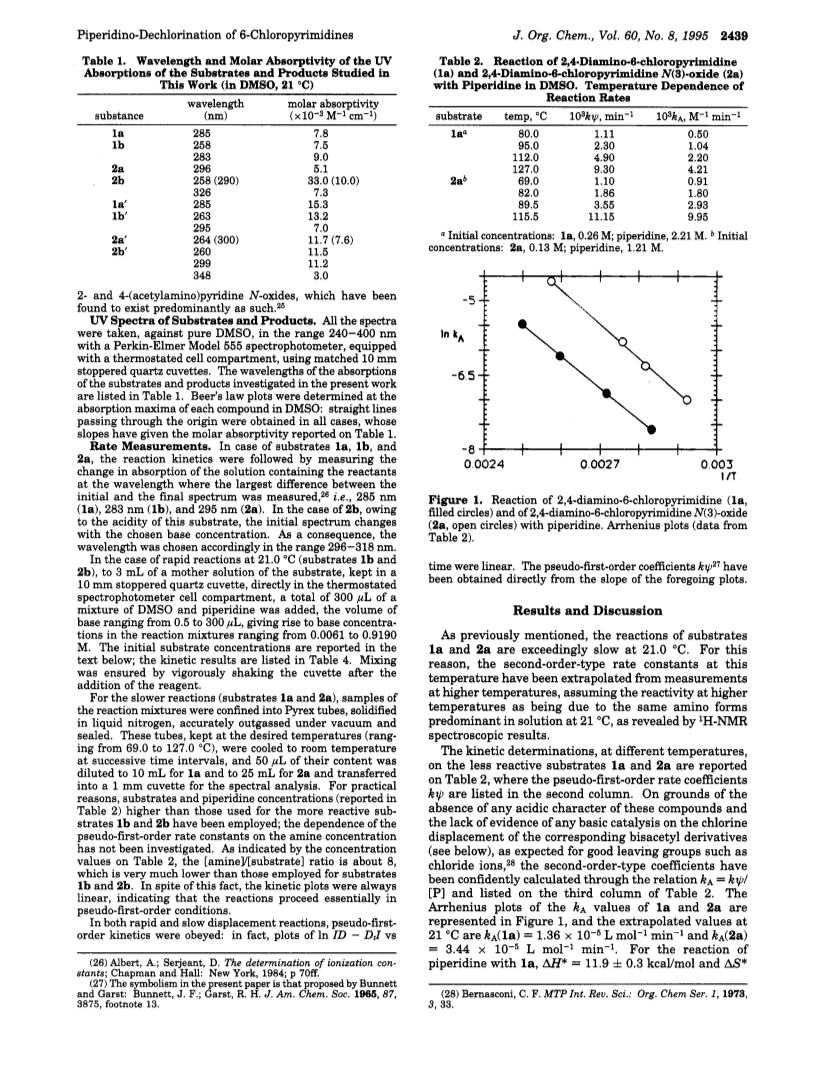
The second-order rate constants kA for the piperidino-dechlorination of 2,4-diamino-6-chloropyrimidine (1a), 2,4-bis(acetylamino)-6-chloropyrimidine (1b), 2,4-diamino-6-chloropyrimidine N(3)-oxide (2a), and 2,4-bis(acetylamino)-6-chloropyrimidine N(3)-oxide (2b) have been determined from the corresponding pseudo-first-order rate constants, kψ, measured in DMSO at 21.0 deg C by the UV spectrophotometric procedure.The second-order rate coefficients of the less reactive substrates 1a and 2a at 21 deg C have been obtained as extrapolated values from Arrhenius plots of kA values, calculated through the psudo-first-order-type relationship, kψ = kA
(where
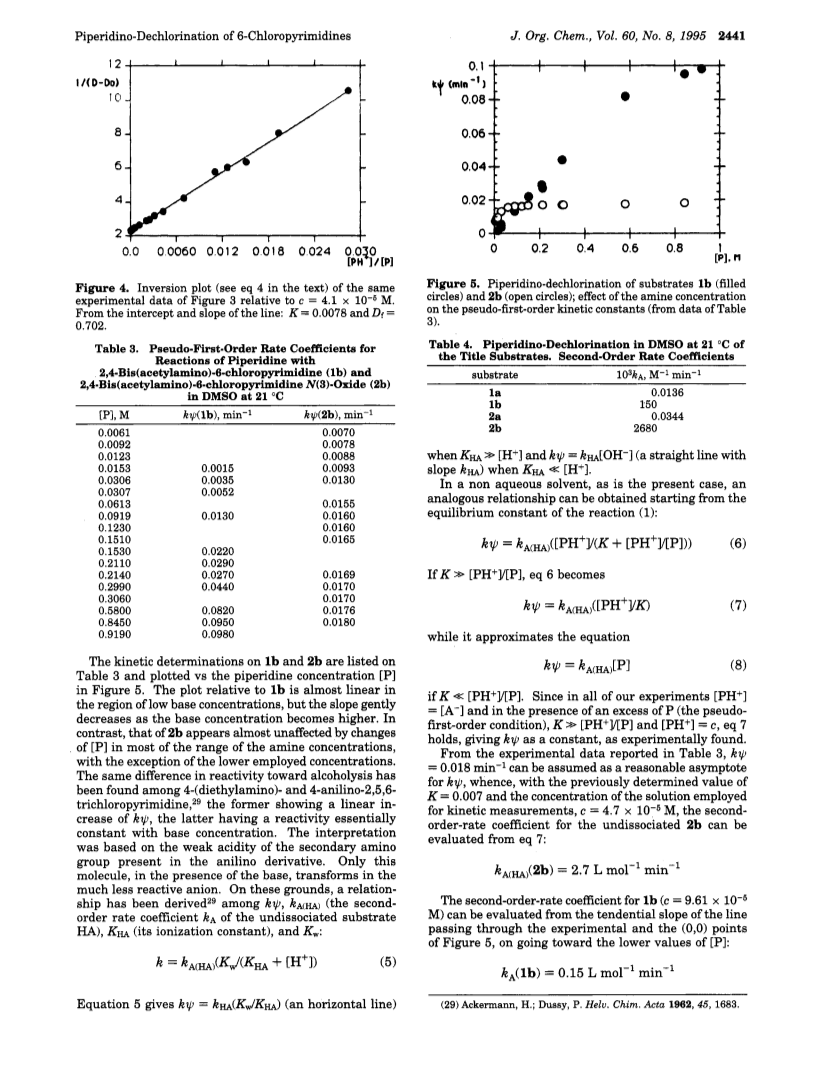
is the amine concentration),from the kψ measured at higher temperatures (kA(1a) = 1.36E-5 and kA(2a) = 3.44E-5 L mol-1 min -1).The reactivities of the acyl derivatives 1b and 2b are remarkably higher than that of the parent compounds 1a and 2a.The pseudo-first-order-rate constants of the more reactive substrates 1b and 2b, measured as a function of piperidine concentration, increase linearly for 1b, with a decreasing curvilinear slope only in the higher concentration region of base; in contrast, the reactivity of 2b remains almost constant and lower than that of 1b for most of the employed base concentrations.This behavior is due to the acidic character of compound 2b, which is almost totally transformed by excess piperidine into an anionic form, much less reactive than the protonated one toward the nucleophilic attack, even at relatively low base concentrations.Compound 1b is much less acidic than 2b and shows deviations from the second-order-type linear behavior only for the higher base concentrations.The equilibrium constant for the acid-base reaction of 2b with piperidine has been obtained spectrophotometrically (K= 0.007 +/- 0.001), and the second-order rate coefficient kA has been calculated from the constant apparent reactivity kψ by means of the formula kA = kψ in the proximity of the origin (kA(1b) = 0.15 L mol-1 min-1).The results indicate that both the acetylation of the exocyclic -NH2 groups and the oxidation of the cyclic N(3)-atom increase the reactivity of the parent compounds toward piperidinolysis, but that the first modification is much more effective than the second one.The dependence of kψ of 1b and 2b on the amine concentration does not give any evidence for base catalysis, as expected in the model of the intermediate complex mechanism when the leaving group is fast to separate (as the -Cl group is) and/or the complex formation is rate-limiting.





Changzhou BaoKang Pharmaceutical & Chemical Co., Ltd
Contact:(86) 519-88782201 88784080 88785278
Address:Henglin town, changzhou,Jiangsu
Shijiazhuang City Xiehe Pharmaceutical Co., Ltd
Contact:+86-311-80817929
Address:Shangzhuang,Shijiazhuang,China
Hangzhou Fortune Biopharm Co.,LTD.
website:http://www.ftpharm.com
Contact:86-571-87621708
Address:279 Zixuan Road,Xihu District,Hangzhou,Zhejiang(310030),China.
SuZhou Bichal Biological Technology CO.,LTD
Contact:+86-512-68051130
Address:NO.32 huoju road HI-TECH Industrial development zone SuZhou China
Wuhan Konberd Biotech Co., Ltd.
Contact:+86-27-87205925
Address:NO.666, Gaoxin Road, Eastlake High-tech zone
Doi:10.1016/j.tetlet.2005.03.132
(2005)Doi:10.1016/S0022-5347(05)67704-X
(1906)Doi:10.1016/j.bmc.2004.05.008
(2004)Doi:10.1021/jo050493w
(2005)Doi:10.1016/0031-9422(83)80170-8
(1983)Doi:10.1021/ol047676+
(2005)