
Journal of Organic Chemistry p. 315 - 321 (1983)
Update date:2022-08-11
Topics:
 Hoffsommer, John C.
Hoffsommer, John C.
 Glover, Donald J.
Glover, Donald J.
 Burlinson, Nicholas E.
Burlinson, Nicholas E.
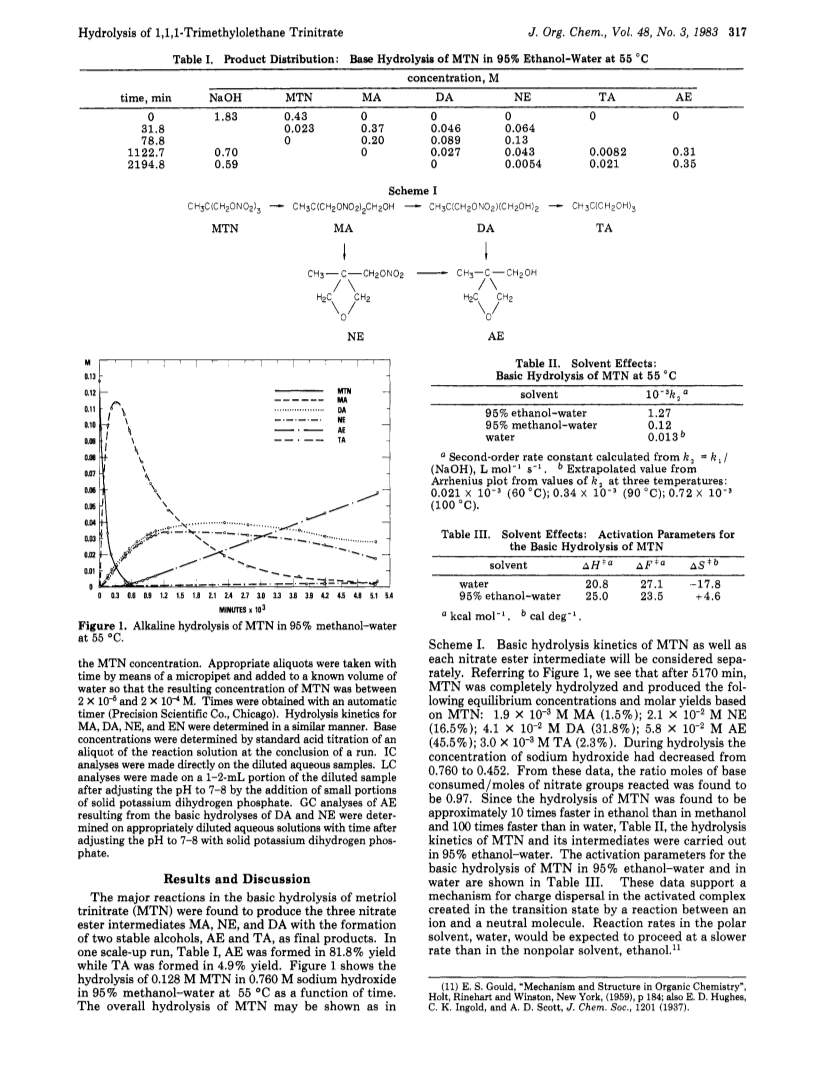
The kinetics for the alkaline homogeneous hydrolysis of 1,1,1-trimethylolethane trinitrate (MTN) in 95percent ethanol-water with sodium hydroxide concentrations between 0.25 and 2.1 M and temperatures between 50.0 and 60.0 degC have been investigated.One mole of MTN was found to react with 3 mol of base and to hydrolize by a series of consecutive and competitive bimolecular and internal cyclization reactions involving three nitrate ester intermediates to form the cyclic alcohol ether 3-methyl-3-oxetanemethanol (AE) as the final major product with only trace amounts of the expected trialcohol 1,1,1-tris(hydroxymethyl)ethane (TA).MTN and its intermediates showed good second-order rate constants for the expression -d(MTN)/dt = k1(MTN) = k2(B-)(MTN), where k1 is the first-order rate constant with excess base, B-.Relative k2 values in 95percent ethanol-water, 95percent methanol-water, and water were found to be 1.0, 0.1, and 0.01, respectively.Hydrolysis kinetics and product formation for each nitrate ester intermediate have been determined, and an overall hydrolysis mechanism for MTN is presented.
View More



Shandong Hongxiang Zinc Co., Ltd
Contact:086-0311-66187879
Address:DaWang developing zone
zhejiang huangyan wanfeng pharm chem co.ltd
Contact:+86-576- 84160728
Address:No. 5 Dazha Road, Economic,Development Zone(JiangKou), Zhejiang, China
Contact:13813902930 025-52714267
Address:20 Fengji Road, Yuhua Economic Development Zone, Nanjing, Jiangsu, P. R. China
Anhui Jiatiansen Agrochemical Co.,Ltd
Contact:15366811918
Address:chemical industrial park,xiangyu town,dongzhi county,anhui,china
Suzhou Ryan Pharmachem Technology Co.,Ltd
Contact:+86-0512-68780025
Address:B-301,No.2 Taishan Road,Suzhou New District,Jiangsu,P.R. China
Doi:10.1007/BF01522061
()Doi:10.1021/ja01332a013
(1933)Doi:10.1016/0009-2614(82)83233-8
(1982)Doi:10.1002/jccs.201600191
(2016)Doi:10.1039/C2971001018a
(1971)Doi:10.1039/C1965000453a
(1965)